~ Introduction ~
The following in a continuation of the subject matter which we posted last week, but was also our broadcast for the week of August 24, 2022. What follows below was broadcast on our August 31, 2022 program and as with the entire 2 part series – was the basis for this intence study, which was conducted in 2014. ~ Editor
 Diagnosis and conventional treatment
Diagnosis and conventional treatment
The diagnosis of prostate cancer relies heavily on the results of a prostate biopsy. A pathologist will analyze the biopsy and assignment a Gleason score or grade. Gleason scores reflect how likely it is that it tumor will spread. The score can vary between 2 and 10 where 2 indicates a small likelihood of spread and 10 indicates a high likelihood of spread. The oncologist will also determine the size of the tumor, if there are cancer cells in nearby lymph nodes or if there is any cancer present in distant tissues (metastasis). The choice of conventional treatment is based on PSA level, Gleason scores, and other information about the prostate tumor. Unfortunately, a negative biopsy (no disease seen) does not exclude the diagnosis of prostate cancer (i.e., a false negative result).
Patients are divided into one of several categories: very low risk, low risk, intermediate risk, high risk, locally advanced, and metastatic prostate cancer. In older patients with very low risk disease, conventional treatment may not be used; instead, doctors provide active surveillance or simply watch for signs of progression.(70) Depending on their individual risk, men with prostate cancer may be treated with active surveillance, radiation therapy, surgery, hormonal therapy, and chemotherapy. Some oncologists may offer other prostate cancer therapies including cryotherapy and high-intensity focused ultrasound or HIFU.
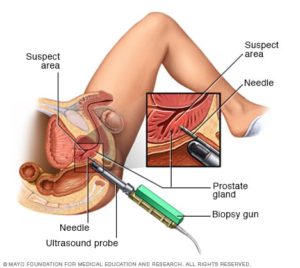 Radiation therapy is usually administered in one of two forms: external beam radiation or brachytherapy. External beam radiation involves a device placed on the outside of the body that projects a beam of ionizing radiation at the prostate gland. Brachytherapy involves the placement of small “seeds” or pellets in the prostate gland itself. These pellets emit low levels of radiation that affect the prostate tissue immediately surrounding each pellet. Since cancer cells grow and reproduce faster than healthy cells, the radiation disrupts prostate cancer more so than normal prostate cells. External beam radiation may cause inflammation of the bladder and the large intestine and it may also increase the risk for bladder and gastrointestinal cancers. While brachytherapy is less likely to cause adverse effects because the total dose of radiation is less and effects smaller areas of tissue, there are still side effects. Brachytherapy is also known to increase the risk of bladder cancer.
Radiation therapy is usually administered in one of two forms: external beam radiation or brachytherapy. External beam radiation involves a device placed on the outside of the body that projects a beam of ionizing radiation at the prostate gland. Brachytherapy involves the placement of small “seeds” or pellets in the prostate gland itself. These pellets emit low levels of radiation that affect the prostate tissue immediately surrounding each pellet. Since cancer cells grow and reproduce faster than healthy cells, the radiation disrupts prostate cancer more so than normal prostate cells. External beam radiation may cause inflammation of the bladder and the large intestine and it may also increase the risk for bladder and gastrointestinal cancers. While brachytherapy is less likely to cause adverse effects because the total dose of radiation is less and effects smaller areas of tissue, there are still side effects. Brachytherapy is also known to increase the risk of bladder cancer.
In men who have metastatic prostate cancer, a special form of radiation may be administered intravenously. Radium-223 moves from the bloodstream into bones, a common site for metastatic prostate cancer cells. The radium-223 is administered in a series of six injections, one per month. Radium-223 emits radiation that kills prostate cancer cells that have migrated to bones.
The traditional surgery for prostate cancer is a radical prostatectomy, which is the complete removal of the prostate gland. Unfortunately, impotence is common after removal of the prostate gland, especially in patients with certain risk factors.(71) The CyberKnife system allows a surgical oncologist to provide radiosurgery, which is closer radiation therapy than true surgery. The CyberKnife system was cleared by the FDA in 2001 for use on any target organ in which radiation therapy is appropriate and indicated. The physician uses advanced imaging technologies and robotics to selectively direct high doses of radiation at prostate cancer tissue. The radiation is delivered from outside the body, so it is noninvasive and no hospital stay is required. Effectiveness of CyberKnife is comparable to other primary treatments for prostate cancer.(72) Men with low and intermediate risk prostate cancer may be candidates for CyberKnife treatment.
Other procedures for prostate cancer treatment – cryotherapy and high-intensity focused ultrasound – are still under clinical evaluation. Cryotherapy and high-intensity focused ultrasound are forms of ablation therapy which selectively destroy tissue either by freezing or by heating the tissue with ultrasound energy, respectively. Cryotherapy is performed under spinal/epidural anesthesia rather than general anesthesia and is less invasive than open surgery to remove the entire prostate gland.
Cryotherapy is an option for men with localized disease or recurrent prostate cancer that remains near the prostate. Early use of cryotherapy was associated with very high complication rates and adverse events and was only offered by few surgical oncologists. However, the techniques associated with cryotherapy have improved, especially the addition of high precision transrectal ultrasound imaging, which allows the surgeon to view the prostate in real time and control the amount of tissue freezing. Because of these advancements, cryotherapy may be a reasonable choice for some men. Candidates for cryotherapy include men with early stage prostate cancer who have not yet had specific treatment such as radiation therapy or surgery. On the other hand, men may be offered cryotherapy as a palliative therapy if other treatments for prostate cancer have failed(73) Cryotherapy may cause pain and swelling in the treated region, incontinence or difficulty with urination, and impotence.
High-intensity focused ultrasound is an outpatient procedure that takes about two hours. The ultrasound device is placed in the rectum near the prostate gland and ultrasound energy heats the cancerous tissue and surrounding tissues to small degree,which destroys the tissue. High-intensity focused ultrasound is not approved in the United States for the treatment of prostate cancer but clinical trials in the United States are ongoing. The technology has been approved for the treatment of prostate cancer in Europe and Asia.
Chemotherapy and hormone therapy are usually reserved for prostate cancer patients with high risk of disease. The hormone therapy for advanced prostate cancer is androgen deprivation therapy, which essentially means blocking or removing the effect of testosterone. Androgen deprivation therapy is accomplished by administering hormonal drugs such as leuprolide or goserelin plus flutamide or by surgically removing the testicles. Bicalutamide (Casodex) is an antiandrogen drug and is apparently ineffective in treating localized prostate cancer. The Early Prostate Cancer program was a randomized, placebo-controlled, international trial with over 8,000 participants comparing bicalutamide or placebo added to standard prostate cancer treatment. After seven years of follow-up, bicalutamide provided no added benefit to men with localized prostate cancer, though men with locally advanced prostate cancer enjoyed significant clinical benefits.(74) Chemotherapy is not often used in prostate cancer, but if it is used, it may cause nausea, vomiting, hair loss, anemia, and deficiencies in the immune system.
~ Protective Factors ~

Coffee is more than just an enjoyable treat, it can actually help to prevent the onslaught of dementia, scientists claim
Coffee
Coffee but not necessarily caffeine, protects against developing lethal (fatal or metastatic) prostate cancer. The Health Professionals Follow-up Study followed nearly 48,000 men over a period of 20 years and found just over 5000 men who developed prostate cancer. Of these, 642 men died or had metastatic prostate cancer. After controlling for other known prostate cancer risk factors, people who consumed more coffee per day were less likely to be in that group of 642(75) most interestingly, decaffeinated coffee had the same effect, which means the protective ingredient is found within coffee, but is not caffeine.
 Soy
Soy
One of the reasons that Asian populations have far fewer cases of prostate cancer than Western cultures could be because many Asian nations have diets high in soy and soybean products. Soybeans are rich sources of healthful substances such as beta-sitosterol, stigmasterol, genistein, daidzein, and glycitein. In a group of 200 men with benign prostatic hyperplasia, those who consumed 20 mg of beta-sitosterol three times a day increased urine flow through the prostate gland and significantly reduced the amount of urine remaining in the bladder compared to those taking placebo.(76) Daidzein and genistein, two other substances in soy, have similar effects on urine flow through the prostate gland. (77)
Genistein, in particular, has a number of anticancer properties: it has been shown to inhibit the growth of prostate cancer cells and cause abnormal cells to undergo apoptosis (“cell suicide”). (78) Animal studies have suggested a diet high in genistein and daidzein results in a lower incidence of prostate cancer and longer disease-free periods after exposure to cancer-causing agents.(79) Among a cohort of 12,395 California Seventh-Day Adventist men, 225 developed prostate cancer during the study period. Those men who frequently consumed soymilk reduced their rate of prostate cancer by 70%.(80) In a survey of 1,619 men of various ethnicities, those who ate soy products had reduced risk of prostate cancer.(81) Indeed, levels of genistein in the blood inversely correlated with risk of prostate cancer in 14,203 Japanese men, i.e., higher blood levels of genistein were associated with lower prostate cancer risk and vice versa.(82) Across various clinical studies, those who commonly ate soy and soy food products lowered their risk of prostate cancer by
30%. (83)
Typical Western diets provide only about 80 mg/day of phytosterols (the useful ingredients in soy) compared to a traditional Japanese diet, which contains about 400 mg/day (84) A three-and-a-half ounce serving of soybeans or tofu contains about 90 mg of beta-sitosterol. Asian cultures are believed to consume about 20-80 mg/day of genistein, whereas Western populations consume about 2-3 mg/day. (77,79)

The deeper red the tomato, the more lycopene it generally contains which is thought to help reduce the incidence of many cancers
Lycopene
Lycopene is a potent antioxidant found in high concentrations in tomatoes, tomato products, and certain other fruits and vegetables (e.g., watermelon, pink grapefruit, carrots, and green peppers). In addition to its antioxidant activity, lycopene has several anticancer effects. In laboratory studies, lycopene can slow the growth and proliferation of prostate cancer cells, can cause prostate cells to commit suicide (apoptosis), stop the cycle of new cancer cells from forming, and prevent tumor cells from spreading through tissues to the rest of the body.(85) Men with the highest levels of lycopene in their blood were least likely to develop prostate cancer.(86,87) A prospective cohort study of 47,894 subjects found lycopene intake from tomato products was inversely associated with the risk of prostate cancer. (88) Likewise, eating tomato sauce decreased the risk of prostate and other forms of cancer. (89,90) A prospective study showed two or more servings of tomato products or lycopene per week reduced the risk of developing prostate cancer by at least 23% compared to those who ate tomato products/lycopene less than once per month.(91) In a study that examined 49,898 male health professionals over a period of 13 years, higher dietary intake of lycopene was associated with lower risk of deadly prostate cancer.(92) Moreover, those that did develop prostate cancer had less angiogenesis (blood vessel connections) in the tumor. Tumors with fewer blood vessel connections do not grow as quickly and are less likely to spread.

Cruciferous vegetables, like cauliflower, have substances which help stop cells from DNA damage
Cruciferous vegetables
Cruciferous vegetables are vegetables such as cauliflower, cabbage, broccoli, bok choy, kale, brussels sprouts, collard greens, and various others. Cruciferous vegetables contain a number of molecules that are protective or potentially protective against prostate cancer, and block normal prostate cells from converting into cancer cells and also slow the rate of prostate cell growth and proliferation.(93) Interestingly, cruciferous vegetables disrupt intracellular signaling pathways in prostate cancer cells but not in healthy prostate gland cells. (93) Thus, these green vegetables are likely to be helpful in prostate cancer prevention but also may help slow the growth of prostate cancer once it exists. In a meta-analysis that included seven cohort and six population-based case-control studies, researchers found prostate cancer risk significantly decreased as intake of cruciferous vegetables increased.(94) On average, the reduced risk was between 10 and 20%. In men who have already developed prostate cancer, those who consumed the highest amounts of cruciferous vegetables decreased the risk of prostate cancer progression by 60% compared to those who consumed the fewest cruciferous vegetables. (95)
 Green Tea
Green Tea
Green tea inhibits prostate cancer cell growth. Studies in rats showed that compounds contained in green tea inhibit the activity of 5-alpha-reductase, the enzyme that converts testosterone to dihydrotestosterone (DHT).(96) DHT has carcinogenic effects on the prostate gland. Researchers have found the most potent of the green tea compounds is a catechin called epigallocatechin-3-gallate (EGCG). Aolyphenone-60, an extract of green tea that is rich in catechins, slowed the development of prostate cancer.(97) Green tea catechins, including EGCG, suppressed the growth of human prostate cancer cells, and prompted them to “commit suicide” (apoptosis). (98) The polyphenolic fraction of green tea not only inhibited localized prostate cancer growth but also inhibited the metastasis of the cancer to distant sites.(99) Interestingly, green tea catechins tend to concentrate in the prostate gland after humans consume green tea suggesting these molecules target the prostate.(100) Clinical trials in humans with green tea have been difficult because of differences in dosages and intake levels. As a result, observational studies have not shown a strong connection between green tea consumption in the prevention of prostate cancer. On the other hand, several phase 2 clinical trials have shown green tea extracts can inhibit prostate cancers from progressing from a precancerous state to a malignant state.(101) According to some reviews, the optimal amount of green tea intake in a day is between 3 to 5 cups, which should be sufficient to supply at least 250 mg of green tea catechins per day. (102)
 Garlic
Garlic
Allium species include various forms of garlic, onion, leak, and chives. Allium vegetables are good source of molecules with cancer fighting properties, such as organic sulfur and flavonols. A compound in aged garlic significantly inhibited the growth of human prostate cancer cells in a laboratory setting. (103,104) A substance within garlic called diallyl disulfide may be the key factor that causes prostate cancer cells to enter apoptosis (programmed cell death; “cell suicide”).(105) The consumption of various Allium species and in particular garlic is associated with a reduced rate of developing prostate cancer.(106) Researchers combined the results of nine epidemiological studies which together included 132 to 192 participants. They found garlic consumption reduced the risk of developing prostate cancer by 23% and onion consumption reduce that risk by 16% (the onion result was not statistically significant). (106)
 Ginger
Ginger
Ginger is a root that is best known as a food substance; however, it possesses a number of interesting biological properties and has been used as a medicine for over two thousand years.(107) Ginger is an anti-inflammatory and antioxidant, which make it useful in the prevention of cancer initiation and development.(107,108) Various compounds extracted from ginger work together to block the proliferation of human prostate cancer cells in test tubes. (109) Extracts of ginger can block the cell cycle of prostate cancer cells and induce those cells to die through apoptosis (“cell suicide”). (108) Animals with prostate cancer fed 100 mg per kilogram body weight of ginger extracts experienced a 56% slowing of their prostate tumor growth as measured by prostate size after eight weeks of treatment.(108) Human trials of ginger root and/or Ginger root extract in the treatment or prevention of prostate cancer have not yet been performed.
 Curcumin
Curcumin
Curcumin, a compound found in turmeric and present in various curry preparations, is currently being evaluated in clinical trials for various forms of cancer, skin and joint disorders, and Alzheimer’s disease. In mice, curcumin has shown remarkable ability to block cancer affects in a variety of cancers.(110) Curcumin decreased the proliferation of both testosterone and testosterone prostate cancer cells in mice.(111) Curcumin also increased the tumor killing power of chemotherapeutic agents in test tubes. (112) Clinical trials of curcumin treatment for prostate cancer have not yet been completed.
 Shiitake and Maitake mushrooms
Shiitake and Maitake mushrooms
Both Shiitake and Maitake mushrooms have been investigated as medicinal foods. Laboratory investigation suggests Shiitake and Maitake mushrooms may be able to boost the immune system and therefore could be helpful in treating conditions such as existing cancers and HIV/AIDS. Both forms of mushrooms have been used in Traditional Chinese Medicine for nearly 2000 years. In Western medicine, specific extracts of these mushrooms have been the focus of most studies. The two substances within shiitake mushrooms that holds the most promise is a beta glucan/glycan called lentinan or Active Hexose Correlated Compound (AHCC). AHCC is rich in alpha-glucans. In a double-blind, placebo-controlled trial, 21 healthy volunteers received either placebo or 3 g per day of AHCC for four weeks. The 10 volunteers in the AHCC group had a significant increase in the activity of dendritic cells.(113) Dendritic cells create specific immunity and are very useful in fighting cancer. (114)
For Maitake mushrooms, the create key ingredient appears to be the D-fraction extract which is rich in beta 1,3 and 1,6 glucans. D-fraction was able to boost the immune system so that it attacked prostate cancer cells without attacking normal, healthy cells.(115) Most work with these extracts has been done in breast cancer and some work in gastric cancer with relatively little attention paid to prostate cancer. However, since the mushrooms ask act as immune boosters there may be generalizable effects against all forms of cancer.
There have been few clinical trials using these mushrooms or their extracts in prostate cancer patients. One study of 62 men with active prostate cancer treated with a shiitake mushroom extract showed it was ineffective at lowering prostate specific antigen levels after six months of use.(116) Conversely, a trial of 75 patients with metastatic prostate cancer showed that 2 mg per week of lentinan injected into a muscle, improved five year survival from the disease when combined with chemotherapy. (117)
 Boron
Boron
Several lines of evidence suggest the element boron may protect against the development of prostate cancer. In animals with prostate cancer, those that consumed additional amounts of boron experienced nearly 90% reduction in PSA levels and tumor size shrunk by nearly 40%.(118) Interestingly, boron (in the form of boric acid) specifically inhibits the proliferation of prostate cancer cells without affecting normal prostate cells.(119) Boric acid appears to block the ability of prostate cancer cells to release calcium, which stunts their growth. (120)
In a group of 456 men across 63 villages in Turkey, men were first divided by those who lived near and worked in boron mines and those who did not. Then the amount of boron in their urine was measured as an indication of boron exposure. While the rate of prostate cancer did not significantly differ between the high and low exposure groups (probably because of the small number of men in the study) those with high boron exposure at lower prostate specific antigen levels and smaller prostate glands, suggesting that boron may be able to block abnormal prostate gland growth.(121) The National Health and Nutrition Examination Survey examined 8720 men and found that those who had the highest boron consumption in their diets were 54% less likely to develop prostate cancer than men with the lowest boron intake.(122) Likewise, areas of Texas that have high amounts of boron in their groundwater have reduced prostate cancer rates and deaths from prostate cancer than areas with low boron concentrations. (123)
 Quercetin
Quercetin
Quercetin is a flavonoid is a natural yellow pigment that is a natural yellow pigment in fruits and vegetables, though it commonly appears alongside green pigments so that the vegetables that contain quercetin may not appear yellow. Foods that contain particularly high levels of quercetin include capers, dill, fennel, red onion, radicchio, and watercress. Quercetin exhibits a number of remarkable effects on prostate cancer cells in laboratory studies. Quercetin reduces the rate of growth of aggressive prostate cancer cells by nearly 70%.(124) this effect is likely due to the fact that quercetin can increase the expression of tumor suppression genes by more than 50% while almost completely suppressing genes that promote cancer.(124) Other evidence suggests that quercetin can block testosterone receptors on prostate cancer cells thus starving them of the hormone that they need to grow and proliferate.(125) The flavonoid can also interfere with prostate cancer cells as they tried to invade other tissues and migrate through the body (blocks metastasis).(126) A double-blind, placebo controlled trial of 500 mg per day of quercetin is nearing completion and results are expected by the end of 2014.
NOTE: This site does not make specific recommendations for products from any supplier of products, however the Editor does use various products from the above referenced (by image) company depicted within this column. ~ J. Bennett, Editor

Flaxseeds contains lignans which are the main type of phytoestrogens in the Western diet.
Flaxseed
Flaxseed, also known as linseed, is used in an enormous number of food products. It is also used to fortify the diets of livestock. For example, eggs with higher levels of omega-3 fatty acids come from hens who have been fed flaxseed. Various health benefits have been attributed to flaxseed including reduced risk of heart disease, cancer, stroke, diabetes, inflammation and inflammatory diseases, and menopausal hot flashes. A flaxseed-supplemented, fat-restricted diet for six months reduce total cholesterol levels and prostate specific antigen levels(127), and also slowed the rate of prostate gland growth in people with benign prostatic hyperplasia.(127) A later study by the same research group showed flaxseed supplementation without a fat restricted diet can reduce prostate cell proliferation after as little as 30 days.(128) In a group of 147 men with prostate cancer who were waiting surgery, supplementation with lignans found in flaxseed, enterolactone and enterodiol, reduced tumor markers in the prostate cancer.(129) This suggested that flaxseed-derived lignans interfered with cancer cell proliferation. (129)
 Grape seed
Grape seed
Grape seeds and extracts of whole grape seeds contain flavonoids, anthocyanins, phenolic procyanidins, and polyphenols such as resveratrol. Grape seeds are edible, but not palatable and so most people consume grape seed extracts. When 200 mg/kg grape seed extract was fed to mice with prostate cancer it significantly inhibited prostate cancer growth and progression.(130) Similar effects were observed when grape seed extracts (particularly proanthocyanidins) were added to testosterone-sensitive and testosterone-insensitive prostate cancer cells.(131) In the VITAL cohort study that included 35,239 men, grape seed extract was by far the most successful supplement at reducing the risk of prostate cancer. Men who used any amount of grape seed supplements reduced their risk of acquiring prostate cancer by 41%.(132) When the group was further divided into men who were “high users” (≥4 days/week and ≥3 years) the prostate cancer risk reduction was 62%.(132) Unfortunately, the authors of the study did not list the amount of grape seed extract considered a standardized dose. Manufacturers recommend daily doses of grape seed extract between 50 mg and 600 mg, according to the study authors.
Modified Citrus Pectin
Pectin is a carbohydrate that is a collection of hundreds of individual carbohydrate molecules. In its natural state, pectin is a dietary fiber because the body cannot absorb it and it passes through the gastrointestinal system. However, modified citrus pectin has been chemically altered so each pectin molecule is smaller and can pass into the blood stream. Modified citrus pectin inhibits the proliferation of prostate cancer cells and prompted the cells to enter apoptosis (“cell suicide”).(133) Modified citrus pectin may also block prostate cancer cells’ ability to bind to blood vessels, move into other regions of the body, and develop their own blood supply.(134) In other words, the supplement may be able to inhibit prostate cancer metastasis, which has been shown in animal studies.(135) In a small clinical trial of 13 men with prostate cancer who had failed to respond to traditional treatment (i.e., prostate removal surgery, radiation, or ablation), modified citrus pectin slowed the rate at which prostate specific antigen levels doubled in 10 out of 13 men.(136) The PSA doubling rate is a marker for prostate cancer growth(137), hence, slowing the doubling rate indicates modified citrus pectin may have slowed prostate cancer growth and progression.
 Saw Palmetto
Saw Palmetto
Saw palmetto is obtained from the small palm tree called Serenoa repens, which grows in the West Indies and the southeast Atlantic coast of North America. Saw palmetto blocks the conversion of testosterone to DHT in the prostate, which may be helpful in reducing prostate cancer cell number and proliferation.(138) Saw palmetto has also demonstrated anti-estrogenic and receptor site-binding effects(139), increased apoptosis (cell suicide) in prostate cells in the laboratory(140), and also blocked the stimulatory effects of the hormone prolactin on prostate cell proliferation.(141) A component of saw palmetto called myristoleic acid kills prostate cancer cells in test tubes.(141) Another study showed an extract of saw palmetto can inhibit the activity of an enzyme that is important in the development of prostate cancer.(142) A saw palmetto extract has been found to be inhibitor of the enzyme 5alpha-reductase, which is the key factor in the development of prostate cancer.(143) A different study also found saw palmetto inhibits 5alpha-reducase in human prostate cancer cell lines without affecting PSA levels. (140)
Clinical studies of saw palmetto on the risk of prostate cancer have been disappointing thus far. Researchers followed a cohort of 35,171 men for a period of 10 years and found those who used saw palmetto at least once a week had no significant reduction in prostate cancer incidence (roughly 5% lower risk, not statistically significant).(144) However, this study was limited because it included many in the saw palmetto group that may have taken very little saw palmetto. Thus, an effect of the natural product would have been missed because of the study design. Other studies have shown similar results, however. In the VITAL study cohort of 35,239 men, saw palmetto use did not correlate with prostate cancer incidence. (132)
 European mistletoe
European mistletoe
European mistletoe is a plant that grows on various trees in temperate regions and is not to be confused with American mistletoe, which is a holiday decoration. In Europe, European mistletoe is used as an adjunct or palliative therapy for cancer. In fact, mistletoe extract was the most commonly prescribed substance in outpatient oncology clinics in Germany in 2002(145), and was prescribed more often than tamoxifen. Most investigators believe mistletoe lectins are responsible for its positive effect on cancer. Mistletoe lectins induce apoptosis(146) (“cell suicide”) and can stimulate the immune system by increasing the number of neutrophils, lymphocytes, and natural killer cells.(147) The goal of supplementation with European mistletoe is fourfold: to improve quality of life, to boost the immune system, to reduce the negative effects of traditional cancer therapy, and to improve the effects of conventional treatment.(148)
A recent review of European mistletoe therapy in oncology identified 13 prospective trials examining survival duration in cancer and 16 prospective trials that examined quality of life, cancer symptom severity measures, and the ability of the supplement to reduce the negative effects of chemotherapy.(145 ) Six of the 13 survival trials showed European mistletoe extended survival in various cancers, but prostate cancer was not tested. Fourteen of the 16 trials showed European mistletoe extracts had a beneficial effect on qualityof-life and cancer symptoms. European mistletoe extracts are very well tolerated and cause few side effects. To date, clinical trials of European mistletoe in patients with prostate cancer have not been performed. However, laboratory studies show extracts of mistletoe may be able to extend the cancer-killing effects of traditional chemotherapy on prostate cancer cells.(149)
Pygeum africanum
Pygeum africanum (Prunus Africana; African plum; Bitter Almond) is a large tree that grows in high elevations in Africa. South African tribes chewed the bark of this tree to improve symptoms of old man’s disease that was later realized to be benign prostatic hyperplasia. Pygeum africanum is the most commonly used medicine in France for benign prostatic hyperplasia.(150) Pygeum has been shown to be effective in the treatment of benign prostatic hyperplasia in several randomized, controlled trials.(151) Because of the natural products ability to reduce symptoms of prostate enlargement, Pygeum africanum may be useful in symptomatic relief in patients with prostate cancer. Extracts from the bark of the plant improve bladder contractility, have anti-inflammatory activity, block testosterone’s effects on the prostate gland, and restore the prostate’s ability to create and pass secretions.(151,152) Circulating levels of testosterone fueled prostate cancer cell growth and tumors of the prostate can interfere with the prostate gland’s ability to secrete substances. Extracts of Pygeum africanum inhibited growth of prostate cancer cells and prompted the cells to undergo apoptosis (“cell suicide”).(153) Moreover, the extract was able to reduce the incidence of prostate cancer in mice prone to develop the disease.(153) A molecule isolated from Pygeum africanum called N-butylbenzene-sulfonamide (NBBS) may be responsible for the beneficial effects of the tree bark on the prostate. (154)
Cernilton
Cernilton is derived from flower pollen. Like Pygeum africanum, Cernilton has a long and successful history in the treatment of benign prostatic hyperplasia. It is a registered pharmaceutical agent well-known to men in Western Europe, Japan, Korea, and South America. Cernilton can relax smooth muscle tone in the urethra while increasing bladder muscle contraction.(155) A study of 79 men given 63 mg of Cernilton pollen extract daily for 12 weeks experienced an increase in urinary flow rate and decreased amounts of residual urine in the bladder.(156) Men taking the pollen extract reported improvements in urgency, intermittency, delayed voiding, post-void dribbling, prolonged voiding, incomplete emptying, dysuria, and nocturia. Cernilton also blocks the effects of testosterone on the prostate gland(157), which may be helpful both in benign prostatic hyperplasia and prostate cancer. Cernitin is able to selectively block the growth of cancerous prostate cells while not interfering with normal prostate cells.(158) Cernitin cut the growth rate of prostate cancer cells in half.(159) Human trials testing the effect of Cernilton in prostate cancer patients have not yet been performed.
 Licorice root
Licorice root
Licorice root (Glycyrrhiza spp.) has been used as a food and medicinal product in both Eastern and Western medicine. Robust scientific evidence supports the use of licorice root in the treatment of heartburn, but it has been used in conditions as varied as hepatitis to chronic fatigue syndrome to infertility. Extracts of licorice root are able to kill testosterone-sensitive and testosterone-insensitive prostate cancer cells.(160) The extract does so by prompting the cancer cells to undergo apoptosis (“natural cell suicide”). A separate laboratory work suggests that an extract of licorice root, 18α-glycyrrhetinic acid, blocks the inflammation caused by prostate cancer cells.(161) Glycyrrhetinic acid also reduce the production of prostate specific antigen by prostate cancer cells in test tubes.(162) Extracts of the root may also block prostate cancer cells from becoming metastatic and invading other tissues.(163) Mice with prostate cancer that were treated with an extract of licorice called dibenzoylmethane have slower growth and progression of their disease compared to control.(164) Unfortunately, human trials of licorice root have not yet been performed nor have prospective studies examining the effects of Glycyrrhiza on prostate cancer risk.
PC-SPES
PC-SPES is a dietary supplement that contains a number of different herbs (according to the manufacturer it contains Ganoderma lucidium, Dendranthema morifolium, Glycyrrhiza glabra L., Isatis indigotica, Panax  pseudoginseng, Rabdosia rubescens, Scutellaria baicalensis, and Serenoa repens). The supplement can disrupt the growth cycle and proliferation of several types of prostate cancer cells.(165) the authors also report that rats with prostate cancer fed 0.025% and 0.05% diets of PC-SPES exhibited a dose-dependent reduction in the number of tumors and significantly slower tumor growth rate.(165) The multi-herb supplement reduced prostate specific antigen levels in men with prostate cancer.(166) A second clinical study in 16 men with hormone-refractory prostate cancer showed that PC-SPES improve quality of life and reduced pain and prostate specific antigen levels.(167)
pseudoginseng, Rabdosia rubescens, Scutellaria baicalensis, and Serenoa repens). The supplement can disrupt the growth cycle and proliferation of several types of prostate cancer cells.(165) the authors also report that rats with prostate cancer fed 0.025% and 0.05% diets of PC-SPES exhibited a dose-dependent reduction in the number of tumors and significantly slower tumor growth rate.(165) The multi-herb supplement reduced prostate specific antigen levels in men with prostate cancer.(166) A second clinical study in 16 men with hormone-refractory prostate cancer showed that PC-SPES improve quality of life and reduced pain and prostate specific antigen levels.(167)
In a trial that included 33 men with testosterone-dependent and 37 men with testosterone-in dependent prostate cancer, nine capsules a day of PC-SPES reduced prostate specific antigen levels in the dependent group by 80% or more.(168) In this group, 97% of patients had reductions in testosterone equivalent to testicular removal surgery, which is the conventional treatment for prostate cancer in some men.(168) The study authors report severe toxicities occurred. There were three cases of serious blood clots and three other cases of allergic reactions to the compound. In a randomized, phase 2 clinical trial in men with testosteroneindependent prostate cancer, men received three capsules of PC-SPES three times a day or the estrogen diethylstilbestrol. PC-SPES reduced prostate specific antigen levels by 50% or more in 40% of those treated and outperformed diethylstilbestrol. Unfortunately, even though men were on warfarin to thin the blood prophylactically, five people had clotting events (one in the PC-SPES group, and four in the DES group). The herbal supplement PC-SPES has strong pro-estrogen affects, which are likely responsible for its role in prostate cancer. This estrogen effect also increases the risk for blood clots, which needs to be considered before starting treatment.
Inositol Hexaphosphate
Inositol hexaphosphate (IP6) is a found in cereals, soy, and legumes. IP6 strongly inhibits growth of human prostate cancer cells in vitro and, quite interestingly, prompts differentiation of these cells.(169) Cell differentiation is important because it is a m ark of less invasive, less aggressive cancer. In an in vivo study, mice with prostate cancer were fed water with varying concentrations of IP6 (0%, 1%, 2%, or 4%). These mice had been experimentally altered to grow prostate cancer from very early in life. While mice that did not receive any IP6 (0%) grew predictably large prostate tumors as they grew older, mice in the 2% and 4% groups developed much smaller tumors.(170) Moreover, tumors that grew in these IP6-treated groups were less advanced tumors, meaning they had lower Gleason scores.(170) The researchers found that IP6 treated groups had a lack of blood vessels supplying the tumor.(170) This means that IP6 likely has anti-angiogenic effects. IP6 also interfered with the prostate cancer cells ability to absorb glucose – essentially starving the cells of energy.(170) clinical trials of IP6 in cancer patients are needed.
ark of less invasive, less aggressive cancer. In an in vivo study, mice with prostate cancer were fed water with varying concentrations of IP6 (0%, 1%, 2%, or 4%). These mice had been experimentally altered to grow prostate cancer from very early in life. While mice that did not receive any IP6 (0%) grew predictably large prostate tumors as they grew older, mice in the 2% and 4% groups developed much smaller tumors.(170) Moreover, tumors that grew in these IP6-treated groups were less advanced tumors, meaning they had lower Gleason scores.(170) The researchers found that IP6 treated groups had a lack of blood vessels supplying the tumor.(170) This means that IP6 likely has anti-angiogenic effects. IP6 also interfered with the prostate cancer cells ability to absorb glucose – essentially starving the cells of energy.(170) clinical trials of IP6 in cancer patients are needed.
[Specific to Part 2 of this series. Other references are posted within Part 1 of the series.]
70. Mohler J, Bahnson RR, Boston B, et al. NCCN clinical practice guidelines in oncology: prostate cancer. J Natl Compr Canc Netw. Feb 2010;8(2):162-200.
71. Alemozaffar M, Regan MM, Cooperberg MR, et al. Prediction of erectile function following treatment for prostate cancer. JAMA. Sep 21 2011;306(11):1205-1214.
72. King CR, Brooks JD, Gill H, Presti JC, Jr. Longterm outcomes from a prospective trial of stereotactic body radiotherapy for low-risk prostate cancer. Int J Radiat Oncol Biol Phys. Feb 1 2012;82(2):877-882.
73. Sesia G, Ferrando U, Fontana G, Laudi M, Cauda F. Palliative cryotherapy in inoperable prostate carcinoma. Recent Results Cancer Res. 1977(60):84-90.
74. McLeod DG, Iversen P, See WA, Morris T, Armstrong J, Wirth MP. Bicalutamide 150 mg plus standard care vs standard care alone for early prostate cancer. BJU Int. Feb 2006;97(2):247-254.
75. Wilson KM, Kasperzyk JL, Rider JR, et al. Coffee consumption and prostate cancer risk and progression in the Health Professionals Follow-up Study. J Natl Cancer Inst. Jun 8 2011;103(11):876-884.
76. Berges RR, Windeler J, Trampisch HJ, Senge T. Randomised, placebo-controlled, double-blind clinical trial of beta-sitosterol in patients with benign prostatic hyperplasia. Beta-sitosterol Study Group. Lancet. Jun 17 1995;345(8964):1529-1532.
77. Messina M, Barnes S. The role of soy products in reducing risk of cancer. J Natl Cancer Inst. Apr 17 1991;83(8):541-546.
78. Kyle E, Neckers L, Takimoto C, Curt G, Bergan R. Genistein-induced apoptosis of prostate cancer cells is preceded by a specific decrease in focal adhesion kinase activity. Mol Pharmacol. Feb 1997;51(2):193-200.
79. Messina MJ, Persky V, Setchell KD, Barnes S. Soy intake and cancer risk: a review of the in vitro and in vivo data. Nutr Cancer. 1994;21(2):113-131.
80. Jacobsen BK, Knutsen SF, Fraser GE. Does high soy milk intake reduce prostate cancer incidence? The Adventist Health Study (United States). Cancer Causes Control. Dec 1998;9(6):553-557.
81. Kolonel LN, Hankin JH, Whittemore AS, et al. Vegetables, fruits, legumes and prostate cancer: a multiethnic
case-control study. Cancer Epidemiol Biomarkers Prev. Aug 2000;9(8):795-804.
82. Kurahashi N, Iwasaki M, Inoue M, Sasazuki S, Tsugane S. Plasma isoflavones and subsequent risk of prostate cancer in a nested case-control study: the Japan Public Health Center. J Clin Oncol. Dec 20 2008;26(36):5923-5929.
83. Yan L, Spitznagel EL. Meta-analysis of soy food and risk of prostate cancer in men. Int J Cancer. Nov 20 2005;117(4):667-669.
84. Adlercreutz H, Mazur W. Phyto-oestrogens and Western diseases. Ann Med. Apr 1997;29(2):95-120.
85. Holzapfel NP, Holzapfel BM, Champ S, Feldthusen J, Clements J, Hutmacher DW. The potential role of lycopene for the prevention and therapy of prostate cancer: from molecular mechanisms to clinical evidence. Int J Mol Sci. 2013;14(7):14620-14646.
86. Rao AV, Fleshner N, Agarwal S. Serum and tissue lycopene and biomarkers of oxidation in prostate cancer patients: a case-control study. Nutr Cancer. 1999;33(2):159-164.
87. Putnam SD, Cerhan JR, Parker AS, et al. Lifestyle and anthropometric risk factors for prostate cancer in a cohort of Iowa men. Ann Epidemiol. Aug 2000;10(6):361-369.
88. Giovannucci E, Ascherio A, Rimm EB, Stampfer MJ, Colditz GA, Willett WC. Intake of carotenoids and retinol in relation to risk of prostate cancer. J Natl Cancer Inst. Dec 6 1995;87(23):1767-1776.
89. Guttenplan JB, Chen M, Kosinska W, Thompson S, Zhao Z, Cohen LA. Effects of a lycopene-rich diet on spontaneous and benzo[a]pyrene-induced mutagenesis in prostate, colon and lungs of the lacZ mouse. Cancer Lett. Mar 10 2001;164(1):1-6.
90. Agarwal S, Rao AV. Tomato lycopene and its role in human health and chronic diseases. CMAJ. Sep 19 2000;163(6):739-744.
91. Giovannucci E, Rimm EB, Liu Y, Stampfer MJ, Willett WC. A prospective study of tomato products, lycopene, and prostate cancer risk. J Natl Cancer Inst. Mar 6 2002;94(5):391-398.
92. Zu K, Mucci L, Rosner BA, et al. Dietary lycopene, angiogenesis, and prostate cancer: a prospective study in the prostate-specific antigen era. J Natl Cancer Inst. Feb 2014;106(2):djt430.
93. Watson GW, Beaver LM, Williams DE, Dashwood RH, Ho E. Phytochemicals from cruciferous vegetables, epigenetics, and prostate cancer prevention. AAPS J. 2013;15(4):951-961.
94. Liu B, Mao Q, Cao M, Xie L. Cruciferous vegetables intake and risk of prostate cancer: a meta-analysis. Int J Urol. Feb 2012;19(2):134-141.
95. Richman EL, Carroll PR, Chan JM. Vegetable and fruit intake after diagnosis and risk of prostate cancer progression. Int J Cancer. Jul 1 2012;131(1):201-210.
96. Liao S, Hiipakka RA. Selective inhibition of steroid 5 alpha-reductase isozymes by tea epicatechin-3-gallate and epigallocatechin-3-gallate. Biochem Biophys Res Commun. Sep 25 1995;214(3):833-838.
97. Satoh K, Sakamoto Y, Ogata A, et al. Inhibition of aromatase activity by green tea extract catechins and their endocrinological effects of oral administration in rats. Food Chem Toxicol. Jul 2002;40(7):925-933.
98. Chung LY, Cheung TC, Kong SK, et al. Induction of apoptosis by green tea catechins in human prostate cancer DU145 cells. Life Sci. Jan 26 2001;68(10):1207-1214.
99. Gupta S, Hastak K, Ahmad N, Lewin JS, Mukhtar H. Inhibition of prostate carcinogenesis in TRAMP mice by oral infusion of green tea polyphenols. Proc Natl Acad Sci U S A. Aug 28 2001;98(18):10350-10355.
100. Henning SM, Aronson W, Niu Y, et al. Tea polyphenols and theaflavins are present in prostate tissue of humans and mice after green and black tea consumption. J Nutr. Jul 2006;136(7):1839-1843.
101. Yuan JM. Cancer prevention by green tea: evidence from epidemiologic studies. Am J Clin Nutr. Dec 2013;98(6 Suppl):1676S-1681S.
102. Boehm K, Borrelli F, Ernst E, et al. Green tea (Camellia sinensis) for the prevention of cancer. Cochrane Database Syst Rev. 2009(3):CD005004.
103. Raloff J. Radical prostates: Female hormones may play a pivotal role in a distinctly male epidemic. Science News. 1997;151(8):126-127.
104. Pinto JT, Qiao C, Xing J, et al. Alterations of prostate biomarker expression and testosterone utilization in
human LNCaP prostatic carcinoma cells by garlic-derived S-allylmercaptocysteine. Prostate. Dec 1 2000;45(4):304-314.
105. Arunkumar A, Vijayababu MR, Kanagaraj P, Balasubramanian K, Aruldhas MM, Arunakaran J. Growth suppressing effect of garlic compound diallyl disulfide on prostate cancer cell line (PC-3) in vitro. Biol Pharm Bull. Apr 2005;28(4):740-743.
106. Zhou XF, Ding ZS, Liu NB. Allium vegetables and risk of prostate cancer: evidence from 132,192 subjects. Asian Pac J Cancer Prev. 2013;14(7):4131-4134.
107. Shukla Y, Singh M. Cancer preventive properties of ginger: a brief review. Food Chem Toxicol. May2007;45(5):683-690.
108. Karna P, Chagani S, Gundala SR, et al. Benefits of whole ginger extract in prostate cancer. Br J Nutr. Feb 2012;107(4):473-484.
109. Brahmbhatt M, Gundala SR, Asif G, Shamsi SA, Aneja R. Ginger phytochemicals exhibit synergy to inhibit prostate cancer cell proliferation. Nutr Cancer. 2013;65(2):263-272.
110. Rao CV, Rivenson A, Simi B, Reddy BS. Chemoprevention of colon carcinogenesis by dietary curcumin, a naturally occurring plant phenolic compound. Cancer Res. Jan 15 1995;55(2):259-266.
111. Huang MT, Newmark HL, Frenkel K. Inhibitory effects of curcumin on tumorigenesis in mice. J Cell Biochem Suppl. 1997;27:26-34.
112. Hour TC, Chen J, Huang CY, Guan JY, Lu SH, Pu YS. Curcumin enhances cytotoxicity of chemotherapeutic agents in prostate cancer cells by inducing p21(WAF1/CIP1) and C/EBPbeta expressions and suppressing NF-kappaB activation. Prostate. May 15 2002;51(3):211-218.
113. Terakawa N, Matsui Y, Satoi S, et al. Immunological effect of active hexose correlated compound (AHCC) in healthy volunteers: a double-blind, placebo-controlled trial. Nutr Cancer. 2008;60(5):643-651.
114. Palucka K, Banchereau J. Cancer immunotherapy via dendritic cells. Nat Rev Cancer. 2012;12(4):265-277.
115. Pyo P, Louie B, Rajamahanty S, Choudhury M, Konno S. Possible immunotherapeutic potentiation with D-fraction in prostate cancer cells. J Hematol Oncol. 2008;1:25.
116. deVere White RW, Hackman RM, Soares SE, Beckett LA, Sun B. Effects of a mushroom mycelium extract on the treatment of prostate cancer. Urology. Oct 2002;60(4):640-644.
117. Tari K, Satake I, Nakagomi K, et al. [Effect of lentinan for advanced prostate carcinoma]. Hinyokika Kiyo. Feb 1994;40(2):119-123.
118. Gallardo-Williams MT, Chapin RE, King PE, et al. Boron supplementation inhibits the growth and local expression of IGF-1 in human prostate adenocarcinoma (LNCaP) tumors in nude mice. Toxicol Pathol. Jan-Feb 2004;32(1):73-78.
119. Barranco WT, Eckhert CD. Boric acid inhibits human prostate cancer cell proliferation. Cancer Lett. Dec 8
2004;216(1):21-29.
120. Barranco WT, Kim DH, Stella SL, Jr., Eckhert CD. Boric acid inhibits stored Ca2+ release in DU-145 prostate cancer cells. Cell Biol Toxicol. Aug 2009;25(4):309-320.
121. Muezzinoglu T, Korkmaz M, Nese N, et al. Prevalence of prostate cancer in high boron-exposed population: a community-based study. Biol Trace Elem Res. Dec 2011;144(1-3):49-57.
122. Cui Y, Winton MI, Zhang ZF, et al. Dietary boron intake and prostate cancer risk. Oncol Rep. Apr 2004;11(4):887-892.
123. Barranco WT, Hudak PF, Eckhert CD. Evaluation of ecological and in vitro effects of boron on prostate cancer risk (United States). Cancer Causes Control. Feb 2007;18(1):71-77.
124. Nair HK, Rao KV, Aalinkeel R, Mahajan S, Chawda R, Schwartz SA. Inhibition of prostate cancer cell colony formation by the flavonoid quercetin correlates with modulation of specific regulatory genes. Clin Diagn Lab Immunol. Jan 2004;11(1):63-69.
125. Yuan H, Young CY, Tian Y, Liu Z, Zhang M, Lou H. Suppression of the androgen receptor function by quercetin through protein-protein interactions of Sp1, c-Jun, and the androgen receptor in human prostate cancer cells. Mol Cell Biochem. Jun 2010;339(1-2):253-262.
126. Senthilkumar K, Arunkumar R, Elumalai P, et al. Quercetin inhibits invasion, migration and signalling molecules involved in cell survival and proliferation of prostate cancer cell line (PC-3). Cell Biochem Funct. Mar 2011;29(2):87-95.
127. Demark-Wahnefried W, Robertson CN, Walther PJ, Polascik TJ, Paulson DF, Vollmer RT. Pilot study to explore effects of low-fat, flaxseed-supplemented diet on proliferation of benign prostatic epithelium and prostate-specific antigen. Urology. May 2004;63(5):900-904.
128. Demark-Wahnefried W, Polascik TJ, George SL, et al. Flaxseed supplementation (not dietary fat restriction) reduces prostate cancer proliferation rates in men presurgery. Cancer Epidemiol Biomarkers Prev. Dec 2008;17(12):3577-3587.
129. Azrad M, Vollmer RT, Madden J, et al. Flaxseedderived enterolactone is inversely associated with tumor cell proliferation in men with localized prostate cancer. J Med Food. Apr 2013;16(4):357-360.
130. Raina K, Singh RP, Agarwal R, Agarwal C. Oral grape seed extract inhibits prostate tumor growth and progression in TRAMP mice. Cancer Res. Jun 15 2007;67(12):5976-5982.
131. Vayalil PK, Mittal A, Katiyar SK. Proanthocyanidins from grape seeds inhibit expression of matrix metalloproteinases in human prostate carcinoma cells, which is associated with the inhibition of activation of MAPK and NF kappa B. Carcinogenesis. Jun 2004;25(6):987-995.
132. Brasky TM, Kristal AR, Navarro SL, et al. Specialty supplements and prostate cancer risk in the VITamins and Lifestyle (VITAL) cohort. Nutr Cancer. 2011;63(4):573-582.
133. Yan J, Katz A. PectaSol-C modified citrus pectin induces apoptosis and inhibition of proliferation in human and mouse androgen-dependent and- independent prostate cancer cells. Integr Cancer Ther. Jun 2010;9(2):197-203.
134. Glinsky VV, Raz A. Modified citrus pectin antimetastatic properties: one bullet, multiple targets. Carbohydr Res. Sep 28 2009;344(14):1788-1791.
135. Pienta KJ, Naik H, Akhtar A, et al. Inhibition of spontaneous metastasis in a rat prostate cancer model by oral administration of modified citrus pectin. J Natl Cancer Inst. Mar 1 1995;87(5):348-353.
136. Guess BW, Scholz MC, Strum SB, Lam RY, Johnson HJ, Jennrich RI. Modified citrus pectin (MCP) increases the prostate-specific antigen doubling time in men with prostate cancer: a phase II pilot study. Prostate Cancer Prostatic Dis. 2003;6(4):301-304.
137. Denham JW, Steigler A, Wilcox C, et al. Time to biochemical failure and prostate-specific antigen doubling time as surrogates for prostate cancer-specific mortality: evidence from the TROG 96.01 randomised controlled trial. Lancet Oncol. Nov 2008;9(11):1058-1068.
138. Sultan C, Terraza A, Devillier C, et al. Inhibition of androgen metabolism and binding by a liposterolic extract of “Serenoa repens B” in human foreskin fibroblasts. J Steroid Biochem. Jan 1984;20(1):515-519.
139. Carilla E, Briley M, Fauran F, Sultan C, Duvilliers C. Binding of Permixon, a new treatment for prostatic benign hyperplasia, to the cytosolic androgen receptor in the rat prostate. J Steroid Biochem. Jan 1984;20(1):521-523.
140. Ishii K, Usui S, Sugimura Y, Yamamoto H, Yoshikawa K, Hiran K. Extract from Serenoa repens suppresses the invasion activity of human urological cancer cells by inhibiting urokinase-type plasminogen activator. Biol Pharm Bull. Feb 2001;24(2):188-190.
141. Iguchi K, Okumura N, Usui S, Sajiki H, Hirota K, Hirano K. Myristoleic acid, a cytotoxic component in the extract from Serenoa repens, induces apoptosis and necrosis in human prostatic LNCaP cells. Prostate. Apr 2001;47(1):59-65.
142. Vacher P, Prevarskaya N, Skryma R, et al. The Lipidosterolic Extract from Serenoa repens Interferes with Prolactin Receptor Signal Transduction. J Biomed Sci. Oct 1995;2(4):357-365.
143. Anderson ML. A preliminary investigation of the enzymatic inhibition of 5alpha-reduction and growth of prostatic carcinoma cell line LNCap-FGC by natural astaxanthin and Saw Palmetto lipid extract in vitro. J Herb Pharmacother. 2005;5(1):17-26.
144. Bonnar-Pizzorno RM, Littman AJ, Kestin M, White E. Saw palmetto supplement use and prostate cancer risk. Nutr Cancer. 2006;55(1):21-27.
145. Horneber MA, Bueschel G, Huber R, Linde K, Rostock M. Mistletoe therapy in oncology. Cochrane Database Syst Rev. 2008(2):CD003297.
146. Bussing A, Schietzel M. Apoptosis-inducing properties of Viscum album L. extracts from different host trees, correlate with their content of toxic mistletoe lectins. Anticancer Res. Jan-Feb 1999;19(1A):23-28.
147. Hajto T, Hostanska K, Gabius HJ. Modulatory potency of the beta-galactoside-specific lectin from mistletoe extract (Iscador) on the host defense system in vivo in rabbits and patients. Cancer Res. Sep 1 1989;49(17):4803-4808.
148. Kienle GS, Berrino F, Bussing A, Portalupi E, Rosenzweig S, Kiene H. Mistletoe in cancer – a systematic review on controlled clinical trials. Eur J Med Res. Mar 27 2003;8(3):109-119.
149. Weissenstein U, Kunz M, Urech K, Baumgartner S. Interaction of standardized mistletoe (Viscum album) extracts with chemotherapeutic drugs regarding cytostatic and cytotoxic effects in vitro. BMC Complement Altern Med. 2014;14:6.
150. Pygeum africanum (Prunus africanus) (African plum tree). Monograph. Altern Med Rev. Feb 2002;7(1):71-74.
151. Wilt T, Ishani A, Mac Donald R, Rutks I, Stark G. Pygeum africanum for benign prostatic hyperplasia. Cochrane Database Syst Rev. 2002(1):CD001044.
152. Schleich S, Papaioannou M, Baniahmad A, Matusch R. Extracts from Pygeum africanum and other ethnobotanical species with antiandrogenic activity. Planta Med. Jul 2006;72(9):807-813.
153. Shenouda NS, Sakla MS, Newton LG, et al. Phytosterol Pygeum africanum regulates prostate cancer in vitro and in vivo. Endocrine. Feb 2007;31(1):72-81.
154. Papaioannou M, Schleich S, Roell D, et al. NBBS isolated from Pygeum africanum bark exhibits androgen antagonistic activity, inhibits AR nuclear translocation and prostate cancer cell growth. Invest New Drugs. Dec 2010;28(6):729-743.
155. Wilt T, Mac Donald R, Ishani A, Rutks I, Stark G. Cernilton for benign prostatic hyperplasia. Cochrane Database Syst Rev. 2000(2):CD001042.
156. Yasumoto R, Kawanishi H, Tsujino T, et al. Clinical evaluation of long-term treatment using cernitin pollen extract in patients with benign prostatic hyperplasia. Clin Ther. Jan-Feb 1995;17(1):82-87.
157. Talpur N, Echard B, Bagchi D, Bagchi M, Preuss HG. Comparison of Saw Palmetto (extract and whole berry) and Cernitin on prostate growth in rats. Mol Cell Biochem. Aug 2003;250(1-2):21-26.
158. Habib FK, Ross M, Buck AC, Ebeling L, Lewenstein A. In vitro evaluation of the pollen extract, cernitin T-60, in the regulation of prostate cell growth. Br J Urol. Oct 1990;66(4):393-397.
159. Habib FK, Ross M, Lewenstein A, Zhang X, Jaton JC. Identification of a prostate inhibitory substance in a pollen extract. Prostate. Mar 1995;26(3):133-139.
160. Thirugnanam S, Xu L, Ramaswamy K, Gnanasekar M. Glycyrrhizin induces apoptosis in prostate cancer cell lines DU-145 and LNCaP. Oncol Rep. Dec 2008;20(6):1387-1392.
161. Shetty AV, Thirugnanam S, Dakshinamoorthy G, et al. 18alpha-glycyrrhetinic acid targets prostate cancer swells by down-regulating inflammation-related genes. Int J Oncol. Sep 2011;39(3):635-640.
162. Hawthorne S, Gallagher S. Effects of glycyrrhetinic acid and liquorice extract on cell proliferation and prostatespecific antigen secretion in LNCaP prostate cancer cells. JPharm Pharmacol. May 2008;60(5):661-666.
163. Park SY, Lim SS, Kim JK, et al. Hexane-ethanol extract of Glycyrrhiza uralensis containing licoricidin inhibits the metastatic capacity of DU145 human prostate cancer cells. Br J Nutr. Nov 2010;104(9):1272-1282.
164. Khor TO, Yu S, Barve A, et al. Dietary feeding of dibenzoylmethane inhibits prostate cancer in transgenic adenocarcinoma of the mouse prostate model. Cancer Res. Sep 1 2009;69(17):7096-7102.
165. Tiwari RK, Geliebter J, Garikapaty VP, Yedavelli SP, Chen S, Mittelman A. Anti-tumor effects of PC-SPES, an herbal formulation in prostate cancer. Int J Oncol. Apr 1999;14(4):713-719.
166. Geliebter J, Tiwari R, Wu JM. PC-SPES in prostate cancer. N Engl J Med. Feb 18 1999;340(7):567-568.
167. Pfeifer BL, Pirani JF, Hamann SR, Klippel KF. PC-SPES, a dietary supplement for the treatment of hormonerefractory prostate cancer. BJU Int. Mar 2000;85(4):481-485.
168. Small EJ, Frohlich MW, Bok R, et al. Prospective trial of the herbal supplement PC-SPES in patients with progressive prostate cancer. J Clin Oncol. Nov 1 2000;18(21):3595-3603.
169. Shamsuddin AM, Yang GY. Inositol hexaphosphate inhibits growth and induces differentiation of PC-3 human prostate cancer cells. Carcinogenesis. Aug 1995;16(8):1975-1979.
170. Raina K, Ravichandran K, Rajamanickam S, Huber KM, Serkova NJ, Agarwal R. Inositol hexaphosphate inhibits tumor growth, vascularity, and metabolism in TRAMP mice: a multiparametric magnetic resonance study. Cancer Prev Res (Phila). Jan 2013;6(1):40-50.


