 ~ Introduction ~
~ Introduction ~
The prostate is a walnut-sized gland located just beneath the urinary bladder. It is part of the urinary system and the reproductive system. There are passages through the prostate that carry either urine or semen. In fact, the prostate also helps produce semen, the thick fluid that carries sperm cells produced in the testicles. The prostate surrounds the upper part of the urethra, which is the tube that carries urine from the bladder.
Prostate function is regulated by testosterone, the male sex hormone, which is produced primarily in the testicles. Prostate cancer is a cancer that starts in the prostate gland, but may spread to other organs and tissues of the body.
Prostate cancer is the second most common cancer in men worldwide. In 2012, for example, there were 1.1 million cases of prostate cancer and over 300,000 men died from the disease.
In the United States, the National Cancer Institute estimates that 233,000 men will be diagnosed with prostate cancer in 2014 and the disease will kill almost 30,000 American men.(1) Despite research developments and innovations, the annual number of new cases of prostate cancer has remained constant over the past two decades. The lifetime risk of prostate cancer for American men (living in the United States) is one in six.(2)
The risk of prostate cancer is strongly correlated with age, so the risk of prostate cancer increases dramatically between age 40 and age 75.(3) Prostate cancer occurs more often in African Americans than in Caucasian or Hispanic men. For reasons that are unclear, the disease is relatively rare in Asia, Africa, and Latin America. Sadly, prostate cancer tends to be more advanced in African American men at the time of diagnosis. There is considerable evidence that prostate cancer runs in families, but it has been a challenge for researchers to determine the specific genes that cause the disease. To date, the only genes that seem to help predict prostate cancer are those that are used for breast and ovarian cancer, namely BRCA1 or BRCA2 mutations.(4) Importantly, simply having a mutation in BRCA does not necessarily mean that a person will develop prostate cancer.
Prostate cancer screening
Prostate cancer usually grows at a slow rate. In the early stages of the disease, prostate cancer is considered “silent,” which means that it does not cause any noticeable symptoms. This can be a dangerous aspect of the disease because men can have prostate cancer and not know it for months to years. Therefore, screening tests for prostate cancer may be helpful.
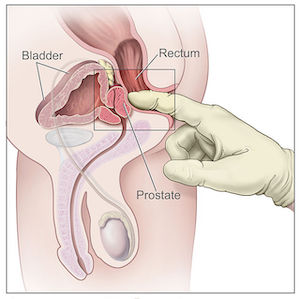 Digital rectal examination
Digital rectal examination
Digital rectal examination is a key screening test for prostate cancer. In a digital rectal examination, the physician places a gloved and lubricated finger into the rectum to feel the shape and size of the prostate gland. Findings that would indicate or suggest prostate cancer during a digital rectal examination are abnormal or asymmetric borders of the prostate gland, hard areas of the prostate called in duration, or nodules in the gland.
While digital rectal examination is important, it is not foolproof. Only 85% of prostate cancers start in the area that they could be felt during the examination. Also, by the time that a prostate tumor has grown to the size that it can be felt by a physician, it is likely to be fairly advanced.(5) Nevertheless, digital rectal examinations can double the chances of detecting prostate cancer(6), so this screening is still important. However, digital rectal examination is likely more accurate when combined with another prostate cancer screening test called prostate specific antigen.(7,8)
Prostate specific antigen (PSA)
Prostate specific antigen, better known as PSA, is a molecule that is produced by the prostate gland. Men with prostate cancer may have increased levels of PSA in their blood because the prostate gland is enlarged and/or the cancer has partially destroyed the prostate gland allowing more PSA to leak into the blood. As with digital rectal examination, there are benefits and limitations to this prostate cancer screening test. On the positive side, PSA levels may be abnormally elevated very, very early in prostate cancer.(9) This means that prostate cancer may be detected long before it has spread and long before it is detectable by digital rectal examination. On the other hand, some illnesses can increase the level of PSA in the blood such as benign prostatic hyperplasia and bacterial prostatitis. Even relatively minor events can raise PSA such as holding onto urine to the point of discomfort, a recent ejaculation, or even digital rectal examination itself.(10,11) Therefore, simply having an abnormally high PSA test does not make the diagnosis of prostate cancer. Tracking PSA levels over time may help physicians distinguish between minor illness and prostate cancer.
Limitations of prostate cancer screening
Neither digital rectal examination nor prostate specific antigen testing are very sensitive or specific for prostate cancer. Because screening can be abnormal in people without prostate cancer. An abnormal PSA and/or digital rectal examination usually requires a prostate biopsy has follow-up. During a prostate biopsy, a needle is passed into the prostate gland and a piece of prostate is removed for analysis. There are risks associated with prostate biopsy including bleeding, infection, and prolonged pain (longer than one week).(12) In a European study, only one man in 1000 actually benefited from more than a decade of PSA testing and 75% of men with abnormal PSA did not have prostate cancer even though they underwent prostate biopsy.(13) Some organizations, such as the United States Preventive Services Task Force have recommended against using PSA for prostate cancer screening.(14) Other groups such as the American Cancer Society and the American Urological Association suggest that prostate screening should be tailored to those individuals that have particular risk such as black men or older man.(15)
 Signs and Symptoms
Signs and Symptoms
As mentioned, a man can have prostate cancer for quite some time before any symptoms are noticeable. However, if symptoms do occur they are usually the result of a prostate that has grown in size because of the cancer. Since the prostate gland rests just below the bladder and urine flows through the gland, swelling in the prostate can interfere with urination.
This means that man may need to urinate more frequently than normal, particularly during the night. Moreover, men with prostate cancer may have difficulty starting urination and stopping urination. In severe cases, a tumor in the prostate gland will block urine flow entirely making urination impossible. Other symptoms of prostate cancer include blood in the urine, blood in the semen, pain during ejaculation, pain or burning during urination, and discomfort or pain in the pelvis, lower back, and/or upper legs.
Some of these symptoms may occur in diseases other than prostate cancer, such as benign prostatic hyperplasia and bacterial prostatitis. Benign prostatic hyperplasia or BPH is very common and causes problems with urination, bacterial prostatitis, on the other hand, is an infection and inflammation of the prostate that causes generalized pain in the pelvis and lower back and may also interfere with urination. Therefore, a man that experiences the symptoms should seek medical attention as soon as possible but the symptoms do not necessarily mean that he has prostate cancer.
Risk factors and protective factors
Research over the past several decades has revealed a number of factors that either increase or decrease the risk of developing prostate cancer. There are four main risk factor groups: age, ethnicity, genetic predisposition, and diet/environment.
Obviously little can be done about the first three risk groups, but overall risk for prostate cancer can be substantially lessened by avoiding certain dietary and environmental risk factors while augmenting and supplementing the diet with potentially beneficial foods and natural products.
Age and genetics
Advancing age is the most potent risk factor for prostate cancer. In fact, prostate cancer is more strongly associated with advancing age than any other human cancer.(3) Diagnosed prostate cancer is very uncommon before the age of 40, though the prevalence of prostate cancer that is not clinically detectable ranges from 2 to 30% in men under the age of 40. The prevalence of prostate cancer peaks between the ages of 70 and 80 with as many as 83% of men in that age range having prostate cancer that may or may not be clinically detectable.(16)
Prostate cancer appears to run in families, but scientists have been unable to identify the gene or genes most often associated with the disease. Several candidates have been identified such as BRCA1, BRCA2, and HOXB13 along with other minor genetic polymorphisms. There are no genetic tests for the diagnosis of prostate cancer. Moreover, no genetic tests reliably predict the risk of developing prostate cancer. Nevertheless, research in this field is ongoing.
Ethnicity
African American men had the highest rates of prostate cancer out of any ethnic group in United States. In a sample of men in their 70s, African American men developed prostate cancer 60% more often than white men. Asian men, on the other hand, develop prostate cancer 30% less often than white men. Another important finding is that African American men generally develop prostate cancer earlier in life than other ethnicities. In a research study of over 12,000 participants under the age of 50, roughly 8% of black men had prostate cancer compared to 3% of white men.(17) It is important to note that genetics may not entirely explain this difference-differences in diet between ethnicities may strongly affect prostate cancer incidence. (18)
Red meat and animal fats
Several studies have shown a diet high in animal fat increases the risk for prostate cancer. (19,20,21) Red meat and processed meat, in particular, are associated with increased rates of prostate cancer.(19) A diet containing few vegetables is a risk factor for prostate cancer(21) and men who consume less than 14 servings of vegetables each week at a 54% increased risk of prostate cancer than men who eat 28 or more servings of vegetables each week. (22)
Omega-3 fatty acids
While omega-3 fatty acids confer a number of health benefits, especially in heart disease, there is evidence to suggest that very high levels of omega-3 fatty acids may actually increase the risk of prostate cancer. Early studies seem to suggest omega-3 fatty acids helped prevent prostate cancer(23), but large, prospective clinical trials show high levels of omega-3 fatty acids can actually increase the risk of high-grade prostate cancer. (24,25) Thus, it seems, that omega-3 fatty acid intake should be modest; it should be high enough to be helpful in heart disease and other forms of cancer but not so high as to increase the risk of prostate cancer.
Corn Oil
Evidence is emerging that the ingestion of large quantities of corn oil may increase the risk of developing prostate cancer and may stimulate the growth of cancer cells once prostate cancer starts. Studies in mice showed corn oil and linoleic acid stimulated the growth of prostate cancer cells.(26,27) It is important to note these studies may implicate high-fat diets as a risk factor for prostate cancer rather than corn oil itself.
Cholesterol
Cholesterol is an important factor in the formation of testosterone, estrogen, and other sexual hormones. Evidence indicates that cholesterol-lowering drugs have a beneficial effect on androgen formation in the prostate. A diet low in saturated fat coupled with regular aerobic exercise can reduce cholesterol levels in most individuals. Likewise, cholesterol lowering drugs called statins may reduce the risk of dying from prostate cancer (28), but they do not seem to reduce the risk of developing prostate cancer. (29,30)
 Cigarette smoking
Cigarette smoking
Smoking cigarettes appears to be a risk factor for the development of prostate cancer but it also worsens the prognosis once prostate cancer is diagnosed. The risk is especially strong in African-Americans. In a study of 1085 men with prostate cancer, those who were heavy smokers had an increased risk of advanced or aggressive prostate cancer than those who were light smokers or never smoked it all. Moreover, the risk was especially strong in African-Americans in this study. Those who smoke at the time of diagnosis are much more likely to have their prostate cancer recur or to die from the cancer than non-smokers. (31)

Alcohol
One large meta-analysis and one large clinical trial showed there is no association between modest alcohol consumption and prostate cancer. The meta-analysis combined the results of 235 studies with a total of 117,000 cases of prostate cancer. The researchers found there was no consistent relationship between alcohol intake and prostate cancer.(32) Likewise, in a prospective study, 10,660 men with no or modest alcohol intake (less than 50 g per day or three alcoholic beverages) had no increased risk for developing prostate cancer. On the other hand, 260 men in the study who consumed four or more alcoholic beverages each day doubled their rate of aggressive (high-grade) prostate cancer. (33)
 Vitamin D, calcium, milk and casein
Vitamin D, calcium, milk and casein
There is a complex association between vitamin D and calcium, milk and the risk of prostate cancer. Several studies have suggested that people who consumed higher levels of dairy products have a greater risk of prostate cancer (34,35,36,37,38), but other studies failed to show the same effect.(39,40) Likewise, some studies have shown that vitamin D deficiency may increase as the risk of prostate cancer,(37,41) while others have indicated high and very low levels of vitamin D increase the risk.(42,43) Based on current evidence, it seems keeping normal levels of vitamin D (not too high or too low) is the best way to minimize risk. One factor that may partially explain the discrepancy and findings is the difference between vitamin D and calcium that comes from milk and vitamin D which comes from supplements. Laboratory evidence suggests casein, a major protein found in cow’s milk cause prostate gland cells to become cancerous and also to promote the growth of these cancerous prostate cells.(44) While there is still more to learn about these substances and prostate cancer it seems the vitamin D and calcium from milk, especially cow’s milk, may be a potential risk factor for prostate cancer. Some sources suggest limiting total calcium (diet and supplements) to 1,500 mg to 2,500 mg per day. (45,46)
 Vitamin E and selenium
Vitamin E and selenium
Numerous laboratory studies showed that the vitamin E was able to inhibit the growth of various types of cancer cells, especially prostate cancer. Moreover, a study of 29,133 men found 1,732 developed prostate cancer during 19 years of follow-up and those who received vitamin E supplementation had a significantly reduced risk of developing prostate cancer.(47) Similar results have been shown for selenium supplementation. A study of 9,345 Japanese-American men who had serum samples drawn and frozen in the 1970s and were assessed for prostate cancer incidence 20 years later revealed those with the highest selenium levels only half as likely to have disease as those with the lowest levels. (48)
Because of these and other studies, researchers performed large, prospective, clinical trials to study the effects of vitamin E and the risk of prostate cancer. The SELECT trial included 35,533 men who were randomly assigned to daily supplementation with 200 mcg (micrograms) of selenium, 400 IU of vitamin E, both, or neither (appropriate placebo). Not only did vitamin E or selenium not prevent prostate cancer, but the trial was stopped early because safety advisers found that people who were supplementing with vitamin E had a significantly increased incidence of prostate cancer. (49,50,51)
In the same trial (SELECT), vitamin E supplementation did not benefit men with low selenium levels but it did increase the risk of aggressive prostate cancer among men with high selenium levels in the blood.(52) In the Physicians’ Health Study II, a double-blind, placebo-controlled trial in which 14,641 male healthcare providers supplemented their diet with one or more vitamins, vitamin E supplementation had no effect on the incidence of prostate cancer (though in this study, vitamin E supplementation did not increase the risk).(53) These results suggest men who are interested in minimizing their risk of prostate cancer should maintain normal levels of vitamin E and selenium but should not exceed normal levels.

Folic acid
Limited evidence suggests folic acid supplementation increases the risk of prostate cancer. This data primarily comes from a large study designed to assess the role of aspirin and folic acid in the prevention of polyps in the large intestine.(54) A group of 1,021 men was divided into two groups and randomly assigned to receive either 1 mg of folic acid each day or placebo. The folic acid was in addition to the amount they may take in through their diet. After 10 years of follow-up, 9.7% of the men who consumed folic acid supplements developed prostate cancer compared to 3.3% in those who took placebo. In other words, folic acid supplements nearly tripled the risk of prostate cancer. It is important to note regular dietary folic acid consumption was not closely monitored in this study and so it is possible men were consuming considerable amounts of folic acid in their diet in addition to folic acid supplements.
 Zinc
Zinc
There are several reasons to believe zinc might be beneficial in preventing prostate cancer, but recent large clinical trials suggest just the opposite. Zinc can reduce the size of the prostate and can decreased symptoms of benign prostatic hyperplasia.(55) In laboratory studies, zinc was able to block the spread of prostate cancer cells and prompted the cells to “commit suicide” (i.e., a process known as apoptosis). On the other hand, the very large Health Professionals Follow-Up Study that followed 46,974 American men found those who consumed over 100 mg of zinc supplements each day for 10 years or more had a 2.29 fold increase in the incidence of prostate cancer. (56)
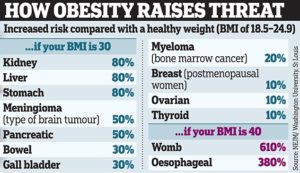 Obesity and inactivity
Obesity and inactivity
Obesity increases the risk of prostate cancer.(57) Moreover, being overweight and obese are risk factors for developing particularly aggressive prostate cancer.(58) The overall increased risk of prostate cancer that results from obesity is relatively small, but the risk increases as the amount of excess weight increases.(59) Also, being obese as a child can increase the risk of prostate cancer later in life.(60,61) A lack of physical activity is a risk factor for prostate cancer, but perhaps surprisingly, the effect only emerges after the age of 65. In other words, the amount of physical activity a man performs does not seem to be related to prostate cancer risk until that man reaches the age of 65. Afterwards, strenuous physical activity for more than three hours per week significantly reduces the risk of advanced or fatal prostate cancer.(62) However, given the benefits of regular exercise throughout life and the fact it might be difficult to start a strenuous exercise regimen at the age of 65, a lifelong dedication to exercise is probably the better health decision.
 Diabetes and excess sugar
Diabetes and excess sugar
Diabetes is a disease in which blood sugar levels become abnormally high because the cells of the body are not able to absorb glucose from the blood. Blood sugar or glucose is a key nutrient for all types of cancer cells, including prostate cancer.
Normal, healthy cells can survive and even thrive on relatively low levels of sugar in the blood. Cancer cells, on the other hand, are highly metabolically active and need a lot of sugar to grow and reproduce. Evidence suggests cancer growth may be faster in people with diabetes, insulin insensitivity, and in those who eat excessive amounts of sugar.(45) Conversely, reducing the amount of simple sugars can slow the growth of prostate cancer cells(45), which may also explain, at least in part, why obesity is a risk factor for prostate cancer. Some studies suggested metformin, a treatment for type II diabetes, might have reduced the risk of prostate cancer. However, in a large study of nearly 120,000 men taking metformin it did not change the incidence of prostate cancer.(63) Nonetheless, in men who have diabetes, are taking metformin, and develop prostate cancer, metformin may be able to improve survival. (64)
Environmental toxins
Certain environmental toxins have been shown to increase the risk of prostate cancer. Exposure to Agent Orange, a chemical that was sprayed in the jungles of Vietnam between 1965 in 1971, increases the rate of aggressive prostate cancers(65) and increases the risk of recurrence after surgery for prostate cancer.(66) Dioxins, found in high concentrations in Agent Orange, are apparently cancer-causing ingredient.

A color-enhanced scanning electron microscope image of prostate cancer cells. A new study suggests that dietary fat may feed prostate tumors and help them spread. Credit Eye of Science/Science Source
Chlordecone is a pesticide used in the West Indies between 1973 and 1993. Exposure to the substance increased the rate of prostate cancer in men. (67) Bisphenol A, widely known as BPA, has been and continues to be used in the manufacture of plastics.
Evidence suggests BPA exposure, especially early in life, increase the risk of developing prostate cancer later in life, though most work has been done in laboratory rather than clinical settings. (68) Other compounds that may increase the risk of prostate cancer include polyhalogenated biphenyls, hexachlorobenzene, and diethylstilbestrol (DES). (69)
~ References ~
1. National Cancer Institute. Surveillance, Epidemiology, and End Results Program: Turning Cancer Data Into Discovery: Prostate Cancer. 2014; http://seer.cancer.gov/statfacts/html/prost.html.
2. Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. Jul-Aug 2011;61(4):212-236.
3. Hankey BF, Feuer EJ, Clegg LX, et al. Cancer surveillance series: interpreting trends in prostate cancer–part I: Evidence of the effects of screening in recent prostate cancer incidence, mortality, and survival rates. J Natl Cancer Inst. Jun 16 1999;91(12):1017-1024.
4. Liede A, Karlan BY, Narod SA. Cancer risks for male carriers of germline mutations in BRCA1 or BRCA2: a review of the literature. J Clin Oncol. Feb 15 2004;22(4):735-742.
5. Chodak GW, Keller P, Schoenberg HW. Assessment of screening for prostate cancer using the digital rectal examination. J Urol. May 1989;141(5):1136-1138.
6. Coley CM, Barry MJ, Fleming C, Mulley AG. Early detection of prostate cancer. Part I: Prior probability and effectiveness of tests. The American College of Physicians. Ann Intern Med. Mar 1 1997;126(5):394-406.
7. Bretton PR. Prostate-specific antigen and digital rectal examination in screening for prostate cancer: a communitybased study. South Med J. Jul 1994;87(7):720-723.
8. Catalona WJ, Richie JP, Ahmann FR, et al. Comparison of digital rectal examination and serum prostate specific antigen in the early detection of prostate cancer: results of a multicenter clinical trial of 6,630 men. J Urol. May 1994;151(5):1283-1290.
9. Gann PH, Hennekens CH, Stampfer MJ. A prospective evaluation of plasma prostate-specific antigen for detection of prostatic cancer. JAMA. Jan 25 1995;273(4):289-294.
10. Tchetgen MB, Oesterling JE. The effect of prostatitis, urinary retention, ejaculation, and ambulation on the serum prostate-specific antigen concentration. Urol Clin North Am. May 1997;24(2):283-291.
11. Yuan JJ, Coplen DE, Petros JA, et al. Effects of rectal examination, prostatic massage, ultrasonography and needle biopsy on serum prostate specific antigen levels. J Urol. Mar 1992;147(3 Pt 2):810-814.
12. Essink-Bot ML, de Koning HJ, Nijs HG, Kirkels WJ, van der Maas PJ, Schroder FH. Short-term effects of population-based screening for prostate cancer on health-related quality of life. J Natl Cancer Inst. Jun 17 1998;90(12):925-931.
13. Schroder FH, Hugosson J, Roobol MJ, et al. Prostatecancer mortality at 11 years of follow-up. N Engl J Med. Mar 15 2012;366(11):981-990.
14. Moyer VA. Screening for prostate cancer: U.S. Preventive Services Task Force recommendation statement.
Ann Intern Med. Jul 17 2012;157(2):120-134.
15. Smith RA, von Eschenbach AC, Wender R, et al. American Cancer Society guidelines for the early detection of cancer: update of early detection guidelines for prostate, colorectal, and endometrial cancers. Also: update 2001–testing for early lung cancer detection. CA Cancer J Clin. Jan-Feb 2001;51(1):38-75; quiz 77-80.
16. Delongchamps NB, Singh A, Haas GP. The role of prevalence in the diagnosis of prostate cancer. Cancer Control. Jul 2006;13(3):158-168.
17. Parker PM, Rice KR, Sterbis JR, et al. Prostate cancer in men less than the age of 50: a comparison of race and outcomes. Urology. Jul 2011;78(1):110-115.
18. Baquet CR, Horm JW, Gibbs T, Greenwald P. Socioeconomic factors and cancer incidence among blacks and whites. J Natl Cancer Inst. Apr 17 1991;83(8):551-557.
19. Sinha R, Park Y, Graubard BI, et al. Meat and meat-related compounds and risk of prostate cancer in a large prospective cohort study in the United States. Am J Epidemiol. Nov 1 2009;170(9):1165-1177.
20. Kolonel LN, Nomura AM, Cooney RV. Dietary fat and prostate cancer: current status. J Natl Cancer Inst. Mar 3 1999;91(5):414-428.
21. Colli JL, Colli A. International comparisons of prostate cancer mortality rates with dietary practices and sunlight levels. Urol Oncol. May-Jun 2006;24(3):184-194.
22. Cohen JH, Kristal AR, Stanford JL. Fruit and vegetable intakes and prostate cancer risk. J Natl Cancer Inst. Jan 5 2000;92(1):61-68.
23. Gerber M. Omega-3 fatty acids and cancers: a systematic update review of epidemiological studies. Br J Nutr. Jun 2012;107 Suppl 2:S228-239.
24. Brasky TM, Till C, White E, et al. Serum phospholipid fatty acids and prostate cancer risk: results from the prostate cancer prevention trial. Am J Epidemiol. Jun 15 2011;173(12):1429-1439.
25. Brasky TM, Darke AK, Song X, et al. Plasma phospholipid fatty acids and prostate cancer risk in the SELECT trial. J Natl Cancer Inst. Aug 7 2013;105(15):1132-1141.
26. Connolly JM, Coleman M, Rose DP. Effects of dietary fatty acids on DU145 human prostate cancer cell growth in athymic nude mice. Nutr Cancer. 1997;29(2):114-119.
27. Lloyd JC, Masko EM, Wu C, et al. Fish oil slows prostate cancer xenograft growth relative to other dietary fats and is associated with decreased mitochondrial and insulin pathway gene expression. Prostate Cancer Prostatic Dis. Dec 2013;16(4):285-291.
28. Yu O, Eberg M, Benayoun S, et al. Use of statins and the risk of death in patients with prostate cancer. J Clin Oncol. Jan 1 2014;32(1):5-11.
29. Platz EA, Leitzmann MF, Visvanathan K, et al. Statin drugs and risk of advanced prostate cancer. J Natl Cancer Inst. Dec 20 2006;98(24):1819-1825.
30. Bonovas S, Filioussi K, Sitaras NM. Statin use and the risk of prostate cancer: A metaanalysis of 6 randomized clinical trials and 13 observational studies. Int J Cancer. Aug 15 2008;123(4):899-904.
31. Kenfield SA, Stampfer MJ, Chan JM, Giovannucci E. Smoking and prostate cancer survival and recurrence. AMA. Jun 22 2011;305(24):2548-2555.
32. Bagnardi V, Blangiardo M, La Vecchia C, Corrao G. A meta-analysis of alcohol drinking and cancer risk. Br J Cancer. Nov 30 2001;85(11):1700-1705.
33. Gong Z, Kristal AR, Schenk JM, Tangen CM, Goodman PJ, Thompson IM. Alcohol consumption, finasteride, and prostate cancer risk: results from the Prostate Cancer Prevention Trial. Cancer. Aug 15 2009;115(16):3661-3669.
34. Chan JM, Giovannucci E, Andersson SO, Yuen J, Adami HO, Wolk A. Dairy products, calcium, phosphorous, vitamin D, and risk of prostate cancer (Sweden). Cancer Causes Control. Dec 1998;9(6):559-566.
35. Gao X, LaValley MP, Tucker KL. Prospective studies of dairy product and calcium intakes and prostate cancer risk: a meta-analysis. J Natl Cancer Inst. Dec 7 2005;97(23):1768- 1777.
36. Giovannucci E, Liu Y, Stampfer MJ, Willett WC. A prospective study of calcium intake and incident and fatal prostate cancer. Cancer Epidemiol Biomarkers Prev. Feb 2006;15(2):203-210.
37. Ma J, Stampfer MJ, Gann PH, et al. Vitamin D receptor polymorphisms, circulating vitamin D metabolites, and risk of prostate cancer in United States physicians. Cancer Epidemiol Biomarkers Prev. May 1998;7(5):385-390.
38. Mitrou PN, Albanes D, Weinstein SJ, et al. A prospective study of dietary calcium, dairy products and prostate cancer risk (Finland). Int J Cancer. Jun 1 2007;120(11):2466-2473.
39. Severi G, English DR, Hopper JL, Giles GG. Re: Prospective studies of dairy product and calcium intakes and prostate cancer risk: a meta-analysis. J Natl Cancer Inst. Jun 7 2006;98(11):794-795; author reply 795.
40. Koh KA, Sesso HD, Paffenbarger RS, Jr., Lee IM. Dairy products, calcium and prostate cancer risk. Br J Cancer. Dec 4 2006;95(11):1582-1585.
41. Ingles SA, Coetzee GA, Ross RK, et al. Association of prostate cancer with vitamin D receptor haplotypes in African-Americans. Cancer Res. Apr 15 1998;58(8):1620-1623.
42. Ahonen MH, Tenkanen L, Teppo L, Hakama M, Tuohimaa P. Prostate cancer risk and prediagnostic serum 25-hydroxyvitamin D levels (Finland). Cancer Causes Control. Oct 2000;11(9):847-852.
43. Tuohimaa P, Tenkanen L, Ahonen M, et al. Both high and low levels of blood vitamin D are associated with a higher prostate cancer risk: a longitudinal, nested case-control study in the Nordic countries. Int J Cancer. Jan 1 2004;108(1):104-108.
44. Melnik BC, John SM, Carrera-Bastos P, Cordain L. The impact of cow’s milk-mediated mTORC1-signaling in the initiation and progression of prostate cancer. Nutr Metab (Lond). 2012;9(1):74.
45. Heber D, Freedland SJ, Jones LW, Nelson WG. Nutrition, Exercise and Prostate Cancer, Duke University; 2009.
46. Prostate Cancer Foundation. Understanding Prostate Cancer: Prevention. http://www.pcf.org/site/c.leJRIROrEpH/b.5802029/k.31EA/Prevention.htm.
47. Weinstein SJ, Wright ME, Lawson KA, et al. Serum and dietary vitamin E in relation to prostate cancer risk. Cancer Epidemiol Biomarkers Prev. Jun 2007;16(6):1253-1259.
48. Nomura AM, Lee J, Stemmermann GN, Combs GF, Jr. Serum selenium and subsequent risk of prostate cancer. Cancer Epidemiol Biomarkers Prev. Sep 2000;9(9):883-887.
49. Lippman SM, Goodman PJ, Klein EA, et al. Designing the Selenium and Vitamin E Cancer Prevention Trial (SELECT). J Natl Cancer Inst. Jan 19 2005;97(2):94-102.
50. Dunn BK, Ryan A, Ford LG. Selenium and Vitamin E Cancer Prevention Trial: a nutrient approach to prostate cancer prevention. Recent Results Cancer Res. 2009;181:183-193.
51. Lippman SM, Klein EA, Goodman PJ, et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA. Jan 7 2009;301(1):39-51.
52. Kristal AR, Darke AK, Morris JS, et al. Baseline selenium status and effects of selenium and vitamin e supplementation on prostate cancer risk. J Natl Cancer Inst. Mar 2014;106(3):djt456.
53. Gaziano JM, Glynn RJ, Christen WG, et al. Vitamins E and C in the prevention of prostate and total cancer in men: the Physicians’ Health Study II randomized controlled trial. JAMA. Jan 7 2009;301(1):52-62.
54. Cole BF, Baron JA, Sandler RS, et al. Folic acid for the prevention of colorectal adenomas: a randomized clinical trial. JAMA. Jun 6 2007;297(21):2351-2359.
55. Kristal AR, Arnold KB, Schenk JM, et al. Dietary patterns, supplement use, and the risk of symptomatic benign prostatic hyperplasia: results from the prostate cancer prevention trial. Am J Epidemiol. Apr 15 2008;167(8):925-934.
56. Leitzmann MF, Stampfer MJ, Wu K, Colditz GA, Willett WC, Giovannucci EL. Zinc supplement use and risk of prostate cancer. J Natl Cancer Inst. Jul 2 2003;95(13):1004-1007.
57. MacInnis RJ, English DR. Body size and composition and prostate cancer risk: systematic review and meta-regression analysis. Cancer Causes Control. Oct 2006;17(8):989-1003.
58. Allott EH, Masko EM, Freedland SJ. Obesity and prostate cancer: weighing the evidence. Eur Urol. May 2013;63(5):800-809.
59. Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. Feb 16 2008;371(9612):569-578.
60. Moller E, Adami HO, Mucci LA, et al. Lifetime body size and prostate cancer risk in a population-based case-control study in Sweden. Cancer Causes Control. Dec 2013;24(12):2143-2155.
61. Giovannucci E, Rimm EB, Stampfer MJ, Colditz GA, Willett WC. Height, body weight, and risk of prostate cancer. Cancer Epidemiol Biomarkers Prev. Aug 1997;6(8):557-563.
62. Giovannucci EL, Liu Y, Leitzmann MF, Stampfer MJ, Willett WC. A prospective study of physical activity and incident and fatal prostate cancer. Arch Intern Med. May 9 2005;165(9):1005-1010.
63. Margel D, Urbach D, Lipscombe LL, et al. Association between metformin use and risk of prostate cancer and its grade. J Natl Cancer Inst. Aug 7 2013;105(15):1123-1131.
64. Margel D, Urbach DR, Lipscombe LL, et al. Metformin use and all-cause and prostate cancer-specific mortality among men with diabetes. J Clin Oncol. Sep 1 2013;31(25):3069-3075.
65. Zafar MB, Terris MK. Prostate cancer detection in veterans with a history of Agent Orange exposure. J Urol. Jul 2001;166(1):100-103.
66. Shah SR, Freedland SJ, Aronson WJ, et al. Exposure to Agent Orange is a significant predictor of prostate-specific antigen (PSA)-based recurrence and a rapid PSA doubling time after radical prostatectomy. BJU Int. May 2009;103(9):1168-1172.
67. Multigner L, Ndong JR, Giusti A, et al. Chlordecone exposure and risk of prostate cancer. J Clin Oncol. Jul 20
2010;28(21):3457-3462.
68. Prins GS, Hu WY, Shi GB, et al. Bisphenol A promotes human prostate stem-progenitor cell self-renewal and increases in vivo carcinogenesis in human prostate epithelium. Endocrinology. Mar 2014;155(3):805-817.
69. Kappas A, Anderson KE, Conney AH, Pantuck EJ, Fishman J, Bradlow HL. Nutrition-endocrine interactions: induction of reciprocal changes in the delta 4-5 alpha-reduction of testosterone and the cytochrome P-450-dependent oxidation of estradiol by dietary macronutrients in man. Proc Natl Acad Sci U S A. Dec 1983;80(24):7646-7649.

