Bacteria in the intestines may prime immune cells to run down tumors.
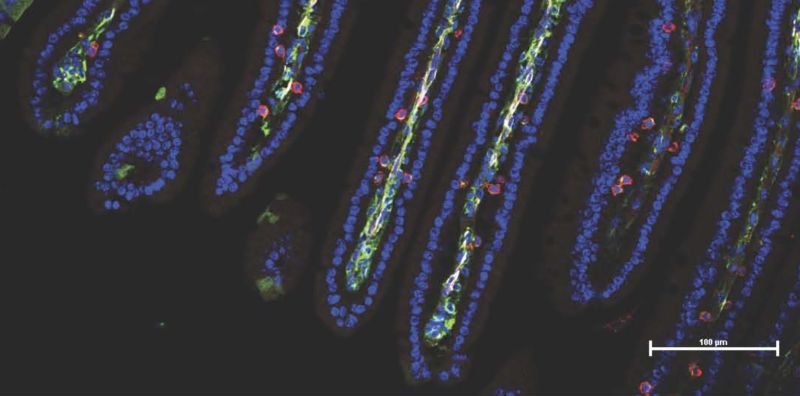
After fecal transplants from responding humans, the gut cells of mice (blue) were flooded with cancer-fighting immune cells (red, green) ~ Dr. Luigi Nezi
New, potent cancer therapies can act like daggers pressed into the hindquarters of the immune system, prodding it to lunge at any cancerous cells in the body. When the drugs work, the immune system tramples tumors into oblivion. But they don’t always work—in fact, cancer drugs can fail 60 to 70 percent of the time. The drugs might not give the immune system a sharp enough sticking in every patient. But according to a pair of new studies, it may not be the immune system that needs a swift kick—it may be the gut.
Some intestinal-dwelling bacteria appear to corral and train immune cells to fight off cancer cells—prior to any spurring from cancer immunotherapies. Without such microbial priming, the drugs may only offer a futile prod. In both studies, published this week in Science, researchers found that the cancer patients who saw no benefit from the drugs (non-responders) were the ones who lacked certain beneficial gut bugs, particularly after taking antibiotics. Meanwhile, cancer patients who did respond to the drugs had bacteria that could prompt the immune system to release chemicals that get cancer-killing immune cells—T cells—to chomp at the bit.
When the researchers transferred the gut microbes from their human cancer patients into germ-free mice with cancer, the rodents mirrored the patients’ fates. That is, mice that got gut microbes from non-responding humans also did not respond to immunotherapies. But, the mice that got microbes from responders responded. And when researchers swapped responder gut microbes into non-responding mice, the mice converted and fought back the cancer.
“Our studies in patients and subsequent mouse research really drive home that our gut microbiomes modulate both systemic and anti-tumor immunity.” That’s according to Jennifer Wargo, a surgical oncologist and geneticist at the University of Texas MD Anderson Cancer Center and the senior author of one of the studies. Dr. Wargo is planning clinical trials to see if fecal transplants in cancer patients could improve immunotherapy success rates.
“You can change your microbiome,” she added. “It’s really not that difficult, so we think these findings open up huge new opportunities.”
Charging ahead
In Dr. Wargo’s study and the other—led by immunologist Laurence Zitvogel of the Gustave Roussy Cancer Campus in Villejuif, France—researchers focused on a type of “checkpoint” inhibitor cancer treatment called “PD-1 inhibitors.” Generally, PD-1 is a protein on the surface of T cells that—in non-cancerous scenarios—acts as a checkpoint to guard against over-zealous immune responses and auto-immune diseases. PD-1 does this by latching onto proteins on healthy cells, namely PD-L1, which basically signals to the T-cell to stand down and not attack the healthy cell.
Crafty cancer cells often don PD-L1, though, allowing them to escape a T cell blitz. That’s where the PD-1 inhibitors come in. If the drugs get in the way of PD-1 binding to PD-L1 on cancer cells, they can help unleash the wrath of T cells on those tumors. But, as mentioned, PD-1 inhibitor therapies often don’t work.
Prior to the new study, Zitvogel and colleagues noticed that recent mouse studies were showing that gut microbes play a role in regulating immune responses to cancers. If that’s true, they hypothesized, then bacteria-killing antibiotics could squash the effects of PD-1 inhibitors. To see if that held up, they simply looked at the outcomes of 249 patients with either lung, kidney, or bladder cancer, some of whom received antibiotics around the time of their PD-1 inhibitor treatments. The researchers found a clear link between antibiotic use and immunotherapy failures. Specifically, the 69 patients taking antibiotics had shorter survival times and periods without their cancers progressing compared with patients with the same cancers and similar health factors.
Next, the researchers examined the communities of microbes in the poop of 100 responding and non-responding cancer patients. They found big differences in the abundance of certain types of bacteria. Specifically, those who responded to PD-1 inhibitors were more likely to carry Akkermansia muciniphila, an intestinal bacterium hypothesized to have anti-inflammatory effects. In mouse experiments, A. muciniphila spurred immune cells to release a chemical signal called IL-2, which is known to regulate T-cells and prime them to attack. Likewise, treatments of A. muciniphila could convert non-responding gut microbes into responding microbes in mice with cancer.
Wargo’s study had similar findings. In their work with 112 skin cancer patients undergoing PD-1 inhibitor treatments, they, too, found that a patient’s gut microbiomes linked with the success or failure of their immunotherapy. Though they didn’t pick out A. muciniphila specifically, they noted that responders tended to have more diverse communities and more of certain types of bacteria. And again, when they transferred the patients’ gut microbiomes into germ-free mice with cancer, the mice met the same fate as their human microbe donors. The researchers also found evidence of beneficial microbes priming T cells.
Together, the studies suggest a big role for gut microbes in determining the cancer-killing potential of immunotherapies. But there are still plenty of questions, namely how, exactly, certain bacteria may spur the immune system to fight cancer and if there are side-effects or potential dangers of manipulating the microbiomes of cancer patients.
Still, as Wargo and colleagues conclude:
These findings highlight the therapeutic potential of modulating the gut microbiome in patients receiving checkpoint blockade immunotherapy, and [they] warrant prompt evaluation in cancer patients through clinical trials.
Written by Beth Mole and published by ARS Technica ~ November 5, 2017.
 FAIR USE NOTICE: This site contains copyrighted material the use of which has not always been specifically authorized by the copyright owner. We are making such material available in our efforts to advance understanding of environmental, political, human rights, economic, democracy, scientific, and social justice issues, etc. We believe this constitutes a ‘fair use’ of any such copyrighted material as provided for in section 107 of the US Copyright Law. In accordance with Title 17 U. S. C. Section 107, the material on this site is distributed without profit to those who have expressed a prior interest in receiving the included information for research and educational purposes. For more information go to: http://www.law.cornell.edu/uscode/17/107.shtml“
FAIR USE NOTICE: This site contains copyrighted material the use of which has not always been specifically authorized by the copyright owner. We are making such material available in our efforts to advance understanding of environmental, political, human rights, economic, democracy, scientific, and social justice issues, etc. We believe this constitutes a ‘fair use’ of any such copyrighted material as provided for in section 107 of the US Copyright Law. In accordance with Title 17 U. S. C. Section 107, the material on this site is distributed without profit to those who have expressed a prior interest in receiving the included information for research and educational purposes. For more information go to: http://www.law.cornell.edu/uscode/17/107.shtml“
