 ‘We didn’t make this medicine for Indians… we made it for western patients who can afford it‘
‘We didn’t make this medicine for Indians… we made it for western patients who can afford it‘
- Indian firm granted government licence to produce copy of a Bayer drug
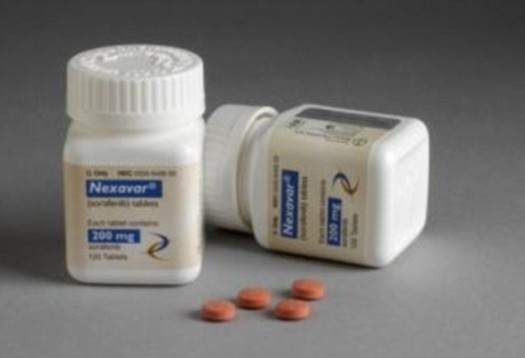
- Nexavar is used for the treatment of kidney, liver and thyroid cancers
- A patient would pay £58,000 for a year’s course of the Bayer version
- However the cost of the Natco version would be around £1,700
- Bayer CEO Marijn Dekkers made comments during a panel discussion
- He has previously described India’s patent laws as ‘essentially theft’
The CEO of phamaceutical giant Bayer has sparked fury after announcing one of the firm’s drugs was for ‘western patients who can afford it’.
Marijn Dekkers made the inflammatory comments after the Indian company Natco Pharma Ltd. were granted a government licence to produce a copy of Bayer’s cancer drug Nexavar which they will sell for 97 per cent less than the original product.
Under Indian law the government grants compulsory licenses to domestic firms to produce copies of drugs if the original isn’t available locally at a reasonable price, regardless of whether they are under patent.
Mr Deekers, who has previously described India’s patent laws as ‘essentially theft’, said: ‘We did not develop this medicine for Indians. We developed it for western patients who can afford it.’
Nexavar, which is also known as Sorafenib, has been approved for the treatment of kidney cancer, advanced liver cancer (hepatocellular carcinoma), and thyroid cancers that are resistant to radioactive iodine treatment.
Currently a kidney cancer patient would pay $96,000 (£58,000) for a year’s course of the Bayer-made drug. However the cost of the Natco version would be around $2,800 (£1,700).
Mr Deekers later posted a comment on a Forbes magazine blog claiming he regretted the way the comment was received.
He wrote: ‘I regret that what was a quick response from me within the framework of a panel discussion at the recent FT Pharma conference has come across in a different way as it was meant by myself.
‘However, I was particularly frustrated by the Indian government’s decision, to not protect a patent on Nexavar that was given to us by the Indian patent authority.
‘I remain firm that there is no excuse for any country to weaken the intellectual property rights. Without new medicines people in developing countries – as well as those in the more prosperous countries – ultimately will all suffer.’
Dr Manica Balasegaram, the Executive Director, Médecins Sans Frontières Access Campaign, which is trying to increase the availability of cancer drugs in developing countries said Mr Dekker’s comments summed up ‘everything that is wrong with the multinational pharmaceutical industry.’
She added: ‘Bayer is effectively admitting that the drugs they develop are deliberately going to be rationed to the wealthiest patients. This is a side-effect of the way drugs are developed today.
‘Pharmaceutical companies are singularly focused on profit and so aggressively push for patents and high drug prices. Diseases that don’t promise a profit are neglected, and patients who can’t afford to pay are cut out of the picture.
‘Drug companies claim to care about global health needs, but their track record says otherwise.’
However other commentators have pointed out that drug companies need to be able to charge high prices in order to continue the vital research and development.
 Published at the Daily Mail, January 24, 2014.
Published at the Daily Mail, January 24, 2014.
FAIR USE NOTICE: This site contains copyrighted material the use of which has not always been specifically authorized by the copyright owner. We are making such material available in our efforts to advance understanding of environmental, political, human rights, economic, democracy, scientific, and social justice issues, etc. We believe this constitutes a ‘fair use’ of any such copyrighted material as provided for in section 107 of the US Copyright Law. In accordance with Title 17 U. S. C. Section 107, the material on this site is distributed without profit to those who have expressed a prior interest in receiving the included information for research and educational purposes. For more information go to: http://www. law. cornell. edu/uscode/17/107. shtml“
