 World Mercury Project gets questions about the number of vaccines given to young children in America today. Different articles cite different numbers and the totals can be confusing. This article and chart are designed to clarify the vaccine schedule and explain why the numbers may not always “add up.” To be accurate, wording about total numbers of vaccines should include phrases like, “may receive up to” or “typically” or “between X and Y” number of vaccines. The individual numbers for any given child will vary depending on the specific vaccines used in his pediatrician’s practice or clinic.
World Mercury Project gets questions about the number of vaccines given to young children in America today. Different articles cite different numbers and the totals can be confusing. This article and chart are designed to clarify the vaccine schedule and explain why the numbers may not always “add up.” To be accurate, wording about total numbers of vaccines should include phrases like, “may receive up to” or “typically” or “between X and Y” number of vaccines. The individual numbers for any given child will vary depending on the specific vaccines used in his pediatrician’s practice or clinic.
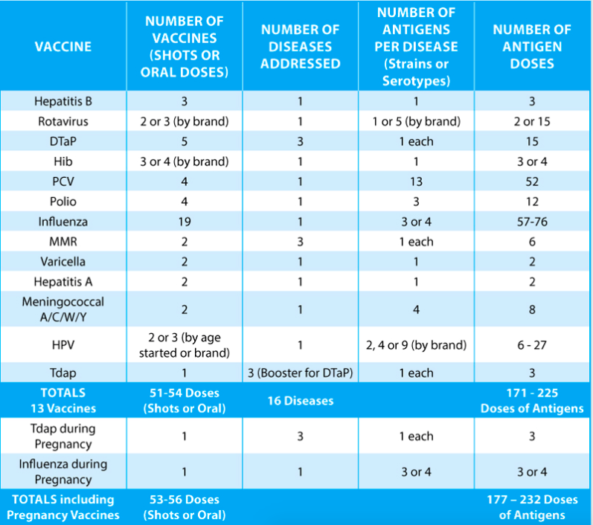
Antigen Totals
To start, there is a difference between the number of vaccines given and the number of antigens given. According to the National Cancer Institute, an antigen is:
Any substance that causes the body to make an immune response against that substance. Antigens include toxins, chemicals, bacteria, viruses, or other substances that come from outside the body. Body tissues and cells, including cancer cells, also have antigens on them that can cause an immune response. These antigens can also be used as markers in laboratory tests to identify those tissues or cells.
In any given vaccine, there will be one or more antigens. A vaccine that contains only one antigen is called monovalent. Examples include the Hiberix, ActHib and PedvaxHib vaccines, each of which contains a single antigen for Haemophilus influenzae type b, a bacterium that can cause meningitis. This single antigen can also, however, be combined into a polyvalent (or multivalent) vaccine such as Pentacel which adds antigens for diphtheria, tetanus, acellular pertussis and inactivated polio; Pentacel contains five antigens in total, hence its name.
A polyvalent or multivalent vaccine can either contain multiple antigens for different diseases (as with Pentacel) or it can contain multiple antigens for different strains or serotypes of the same disease. An example of this would be Pneumovax 23 which contains antigens for 23 different serotypes of pneumococcal bacteria. Annual influenza vaccines can be either trivalent (three strains) or quadrivalent (four strains). The Centers for Disease Control (CDC) and the World Health Organization (WHO) determine the different strains included in the vaccines each flu season based on the strains that are circulating internationally.
While not “officially counted,” some of the other ingredients in vaccines are also antigens. Since they all provoke immune responses, thimerosal, aluminum adjuvants, antibiotics, eggs, saponins and other ingredients should also be considered antigens.
So, if you count the number of injections that a child receives as the number of “vaccines,” the number of vaccines will always be lower than the number of antigens received. The two numbers will never be equal because there are several multivalent vaccines containing multiple antigens that are not available as individual vaccines. The most widely used of these are the MMR (which contains antigens to measles, mumps and rubella viruses), and the DTaP and Tdap vaccines (which contain antigens for diphtheria, tetanus, and pertussis).
Brand Variations
Next, you need to consider how many doses of each vaccine a child receives. This can vary with the brand of vaccine regardless of the number of antigens involved. For example, there are two brands of rotavirus vaccine, Rotarix and RotaTeq. Children receiving Rotarix will be given two doses, while children receiving RotaTeq will be given three doses. These types of variation in dosage are usually based on how many doses were needed to produce sufficient antibody responses in most children in the clinical trials. Once a child has started a vaccine series with one brand, that brand should be used for the remaining vaccines in the series.
Individual Health and History
To further complicate matters, if a child misses a vaccine or a series of vaccines, the “catch up” schedule may be different depending on the child’s age and medical history. To understand this better, it may be helpful to read the four pages of footnotes following the current CDC childhood schedule for 2017. It is also worth noting that the CDC schedule is typically updated once per year—so older articles may cite different numbers of vaccines compared to newer articles.
The Food and Drug Administration’s (FDA) list of all approved vaccines (for children and adults) includes the links to all of their package inserts.
Taking all of this information into account, the summary chart below reflects a typical number of vaccines received by a typical child (up to the age of 18), who receives his or her vaccines on schedule and who has no significant medical conditions that warrant adjustments. The additional information at the bottom of the chart presents possible vaccine exposures while the child’s mom was pregnant.
The bottom line is that kids are getting a lot more vaccines and being exposed to a lot more antigens than they were in the past. The exact number of vaccines is ultimately less important than the overall trend requiring all children to get increasing numbers of vaccines and antigens. CDC has failed to conduct long-term safety studies of the expanding schedule and the combinations of vaccines given concurrently. Parents have a right to demand better science for their children.
 Written by Katie Weisman and the World Mercury Project Team and published by the Collective Evolution ~ December 8, 2017.
Written by Katie Weisman and the World Mercury Project Team and published by the Collective Evolution ~ December 8, 2017.
 FAIR USE NOTICE: This site contains copyrighted material the use of which has not always been specifically authorized by the copyright owner. We are making such material available in our efforts to advance understanding of environmental, political, human rights, economic, democracy, scientific, and social justice issues, etc. We believe this constitutes a ‘fair use’ of any such copyrighted material as provided for in section 107 of the US Copyright Law. In accordance with Title 17 U. S. C. Section 107, the material on this site is distributed without profit to those who have expressed a prior interest in receiving the included information for research and educational purposes. For more information go to: http://www.law.cornell.edu/uscode/17/107.shtml“
FAIR USE NOTICE: This site contains copyrighted material the use of which has not always been specifically authorized by the copyright owner. We are making such material available in our efforts to advance understanding of environmental, political, human rights, economic, democracy, scientific, and social justice issues, etc. We believe this constitutes a ‘fair use’ of any such copyrighted material as provided for in section 107 of the US Copyright Law. In accordance with Title 17 U. S. C. Section 107, the material on this site is distributed without profit to those who have expressed a prior interest in receiving the included information for research and educational purposes. For more information go to: http://www.law.cornell.edu/uscode/17/107.shtml“
